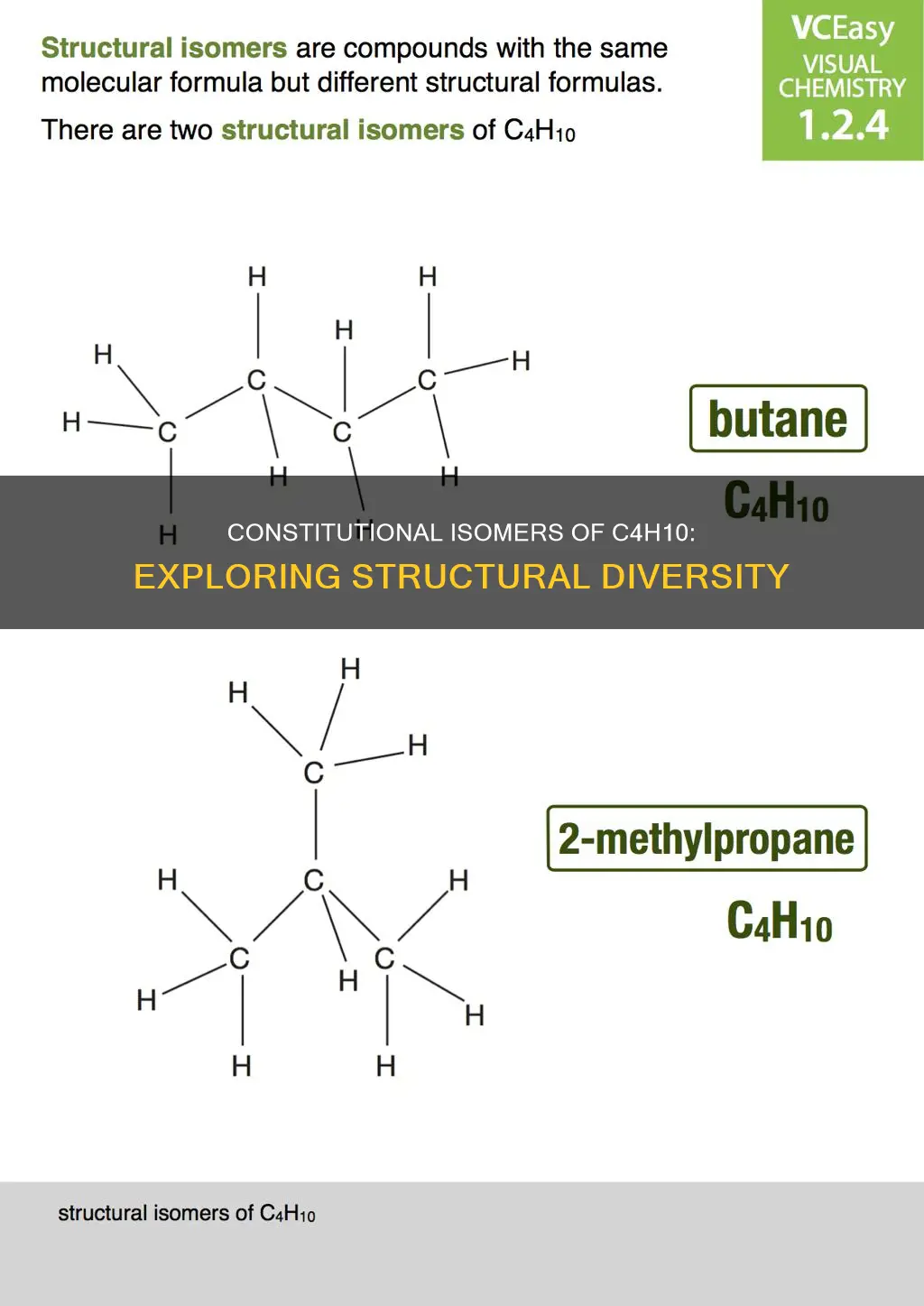
Butane, with the molecular formula C4H10, has two structural isomers: n-butane and isobutane (2-methylpropane). These isomers have the same molecular formula but differ in their carbon bonding arrangements, a classic example of constitutional isomerism. This concept is widely explored in chemistry, where students learn how varying atom connectivity can result in different compounds, despite an identical molecular formula.
| Characteristics | Values |
|---|---|
| Number of Constitutional Isomers | 2 |
| Names of Isomers | n-butane, isobutane (2-methylpropane) |
| Molecular Formula of Isomers | C4H10 |
| Structural Difference | n-butane is a straight-chain compound; isobutane has a branched structure with a side chain |
Explore related products
What You'll Learn
- C4H10 is the molecular formula for butane and its isomers
- n-Butane is a straight-chain compound with four carbon atoms
- Isobutane (2-methylpropane) has a branched structure with one carbon connected to three others
- Constitutional isomers have the same molecular formula but different atom-to-atom connectivity
- Stereoisomerism is when the bonds are the same, but the relative positions of the atoms differ

C4H10 is the molecular formula for butane and its isomers
C4H10 is the molecular formula for butane, a gaseous hydrocarbon consisting of four carbon atoms. Butane has two isomers: n-butane and isobutane (also known as 2-methylpropane). These isomers differ structurally, despite sharing the same molecular formula.
N-butane, or regular butane, is a straight-chain compound with four carbon atoms bonded by single covalent bonds. It is an unbranched structure and is commonly used as a gasoline mixture or in the production of ethylene and butadiene.
On the other hand, isobutane has a side chain in its molecular structure. Specifically, it has three carbon atoms from the parent chain and one carbon atom placed as a side chain at C-2 of the parent chain. This isomerism is known as constitutional or structural isomerism, where the connectivity of atoms differs between molecules.
Constitutional isomers are defined as compounds that share the same molecular formula but have different structural formulas. They exhibit different atom connectivity in their molecules. To identify constitutional isomers, the number of each atom in both molecules must be counted, ensuring the molecular formula remains the same while allowing for different arrangements of atoms.
Butane and its isomers are part of the paraffinic hydrocarbon group and occur naturally in both natural gas and crude oil. They are also produced during petroleum refining.
Voltaire's Ideas: Their Influence on the US Constitution
You may want to see also

n-Butane is a straight-chain compound with four carbon atoms
Butane is an alkane with the molecular formula C4H10, meaning it has four carbon atoms. It exists in two isomeric forms: n-butane and isobutane. These two molecules have the same molecular formula but differ in the arrangement of their atoms, making them constitutional isomers.
N-butane, also known as normal butane, is a straight-chain compound with four carbon atoms bonded by single covalent bonds. In other words, all the carbon atoms in n-butane are linked in a straight chain. This is in contrast to isobutane, which has a branched-chain structure with three carbon atoms in the parent chain and one carbon atom as a side chain attached to the second carbon atom in the parent chain.
The distinction between n-butane and isobutane lies in the connectivity of their carbon atoms. In n-butane, all the carbon atoms are arranged in a linear sequence, forming a straight chain. On the other hand, isobutane exhibits a different connectivity pattern, with one carbon atom branching off the main chain. This structural variation results in distinct molecular shapes for n-butane and isobutane, despite them sharing the same molecular formula.
Constitutional isomers, also known as structural isomers, are compounds that possess the same molecular formula but differ in the way their atoms are connected or arranged. The key characteristic of constitutional isomers is the diversity in the bonding patterns or structural formulas of the atoms within the molecules. To identify constitutional isomers, it is necessary to compare the number of each type of atom in both molecules and ensure they match while also exhibiting different arrangements of atoms.
In summary, n-butane is a straight-chain compound with four carbon atoms, and it is one of the constitutional isomers of C4H10, the other being isobutane. N-butane and isobutane differ in the arrangement of their carbon atoms, resulting in distinct molecular structures despite sharing the same molecular formula.
The Supreme Court's Power: A Constitutional Question
You may want to see also

Isobutane (2-methylpropane) has a branched structure with one carbon connected to three others
Isobutane, also known as 2-methylpropane, is a chemical compound with the molecular formula HC(CH3)3. It is an isomer of butane, which has the molecular formula C4H10. Butane is an alkane with four carbon atoms, and it exists as two isomers: n-butane and isobutane.
The difference between these two isomers lies in their molecular structure. N-butane, or normal butane, is a straight-chain compound with four carbon atoms bonded by single covalent bonds. It is referred to as a "normal" chain because it is unbranched, with no carbon side chains. In contrast, isobutane (2-methylpropane) has a branched structure. It consists of three carbon atoms from the parent chain and one carbon atom attached as a side chain at the second carbon atom (C-2) of the parent chain. This side chain attachment gives isobutane its alternative name, 2-methylpropane.
The presence of a branched structure in isobutane is a key characteristic that distinguishes it from n-butane. This branching occurs because one of the carbon atoms in the chain forms a side chain, resulting in three carbon atoms in the main chain and one carbon atom branching off. This structural difference leads to variations in their chemical properties, despite both molecules having the same molecular formula.
The concept of isomers, such as n-butane and isobutane, falls under the broader category of structural or constitutional isomerism. This type of isomerism involves compounds with the same molecular formula but differing arrangements of atoms within their molecules. The distinct connectivity of atoms in isobutane and n-butane classifies them as constitutional isomers of each other.
Isobutane, due to its branched structure, finds applications in various industries. It serves as a propellant in aerosol spray cans and a refrigerant, offering a functional replacement for certain chlorofluorocarbon and hydrofluorocarbon refrigerants. Additionally, isobutane is used in the petrochemical industry as a precursor molecule for the synthesis of compounds like isooctane.
The Two-Party System: Is It Constitutional?
You may want to see also
Explore related products

Constitutional isomers have the same molecular formula but different atom-to-atom connectivity
Constitutional isomers are compounds that have the same molecular formula but different atom-to-atom connectivity. In other words, they have the same types and amounts of atoms, but these atoms are connected differently, resulting in distinct compounds with different structures and properties. This concept is foundational in the study of isomerism, where isomers are molecules that share the same molecular formula.
To determine if two molecules are constitutional isomers, you can count the number of each atom in both molecules and examine their arrangement. For example, butane (n-butane) and isobutane, both with the molecular formula C4H10, are constitutional isomers. In butane, the carbon atoms form a straight chain, while in isobutane, one carbon atom is branched off the main chain, resulting in different atom connectivity and, consequently, different chemical and physical properties.
Another example of constitutional isomers is cyclohexane (C6H12) and 1-hexene (C6H12), which have the same molecular formula but different connectivities. Cyclohexane has only one way to connect its atoms to form its structure, while 1-hexene also has a unique arrangement of its atoms. However, other compounds, such as 2-hexene and 3-methyl-1-pentene, have multiple ways to connect their atoms, leading to different constitutional isomers.
It is important to distinguish constitutional isomers from stereoisomers, which have the same connectivity of atoms but differ in the arrangement of their atoms in space. Stereoisomers are a type of isomerism where the bonds between atoms are the same, but their relative positions differ. While two molecules can be stereoisomers of each other, they cannot simultaneously be constitutional isomers.
In summary, constitutional isomers share the same molecular formula and composition of atoms but differ in the connectivity and arrangement of these atoms, resulting in distinct compounds with unique structures and properties. This concept is essential in understanding isomerism and the diverse behaviours of compounds with identical molecular formulas.
The Constitution's Journey: A Long Road to Freedom
You may want to see also

Stereoisomerism is when the bonds are the same, but the relative positions of the atoms differ
Butane, with the molecular formula C4H10, has two constitutional isomers: n-butane and isobutane. These two molecules have the same molecular formula but differ in the connectivity of their atoms. In n-butane, all carbon atoms are in a straight chain, while isobutane has a side chain in its molecule.
Now, onto stereoisomerism. Stereoisomerism, also known as spatial isomerism, is a form of isomerism where molecules share the same molecular formula and sequence of bonded atoms (constitution) but differ in the three-dimensional orientations of their atoms in space. In other words, stereoisomerism occurs when the bonds are the same, but the relative positions of the atoms differ.
Stereoisomers can be further categorised into configurational stereoisomers and conformational stereoisomers. A configurational stereoisomer has the opposite configuration at a stereocentre compared to a reference molecule. This means that configurational isomers can only be interconverted by breaking covalent bonds to the stereocentre. On the other hand, stereoisomers that can be converted into one another by rotation around a single bond are called conformational isomers. Alkanes commonly exhibit conformational isomerism due to the presence of C-C bonds. For example, rotating the molecule of butane at the axis of the C-C bond results in eclipsed, gauche, and anti butane conformational isomers.
The terms cis and trans are used to describe the relative positions of substituents on a double bond or a ring. Cis indicates that the substituents are on the same side, while trans means they are on opposite sides. Cis-trans isomerism, also known as geometric isomerism, is a type of stereoisomerism where the stereoisomers differ in the relative positions of substituents on either side of a double bond or on a ring. For example, cis-2-butene and trans-2-butene are conformational isomers that differ in the configuration of their double bond.
Optical isomers, or enantiomers, are a type of stereoisomer that are mirror images of each other but are not superposable. They have the same physical properties, except for how they interact with different enantiomers of other compounds and the direction in which they rotate polarised light. The human hands are a macroscopic example of enantiomers.
The Presidential Cabinet: What the Constitution Says
You may want to see also
Frequently asked questions
There are two constitutional isomers possible for C4H10.
The two constitutional isomers of C4H10 are n-butane and 2-methylpropane (also known as isobutane).
Constitutional isomers, also known as structural isomers, are compounds that have the same molecular formula but different structural formulas. In other words, they have a different connectivity of atoms in their molecules.