There are five constitutional isomers with the molecular formula C6H14. These isomers represent different structural arrangements of the six-carbon alkane without rings or multiple bonds. The five isomers are hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane.
| Characteristics | Values |
|---|---|
| Number of possible structural isomers | 5 |
| Types | Hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane |
Explore related products
$18.99 $19.99
What You'll Learn

There are five constitutional isomers for C6H14
There are indeed five constitutional isomers for the chemical formula C6H14. These isomers are all structural isomers, meaning they represent different arrangements of atoms within the molecule. Specifically, these isomers are all alkanes, which are hydrocarbons that only contain single bonds.
The five isomers are:
- Hexane
- 2-Methylpentane
- 3-Methylpentane
- 2,2-Dimethylbutane
- 2,3-Dimethylbutane
Hexane is a straight-chain alkane, meaning all six carbon atoms are connected in a line. On the other hand, 2-methylpentane and 3-methylpentane are branched-chain alkanes, with a methyl group (CH3) attached to the second and third carbon atoms, respectively. Finally, 2,2-dimethylbutane and 2,3-dimethylbutane have two methyl groups attached to their carbon chains.
The number of possible constitutional isomers increases with the number of carbon atoms. This is because a larger number of carbon atoms allows for more varied connections and structural arrangements.
Mueller Report: What Does Constitution Say?
You may want to see also

These include hexane, 2-methylpentane, 3-methylpentane
There are five structural isomers with the molecular formula C6H14. These include hexane, 2-methylpentane, and 3-methylpentane.
2-methylpentane, also known as isohexane, is a branched-chain alkane and a structural isomer of hexane. It is composed of a methyl group bonded to the second carbon atom in a pentane chain. As of the early 1990s, it was present in American and European gasoline in small amounts. By 2011, its share in US gasoline varied between 2 and 8%. 2-methylpentane has a research octane rating (RON) of 75, a motor octane rating (MON) of 77, and a cetane number (CN) of 29.
Hexane, on the other hand, is a straight-chain alkane with the molecular formula C6H14. It is a highly flammable liquid at room temperature and pressure. Hexane is often used as a non-polar solvent and is commonly found in glue, gasoline, and cleaning agents. It is also used in the extraction of vegetable oils and the production of textiles and plastics.
Unfortunately, I could not find specific information on 3-methylpentane with the molecular formula C6H14. However, as there are five structural isomers of C6H14, it is likely that the other two isomers have similar chemical structures and properties to hexane and 2-methylpentane.
The number of possible constitutional isomers increases rapidly with the number of carbon atoms. This is because each additional carbon atom provides more opportunities for branching and different structural arrangements. In the case of C6H14, there are five unique structural isomers, including hexane, 2-methylpentane, and 3-methylpentane, each with distinct chemical properties and applications.
Schenck v US: Freedom of Speech Violation
You may want to see also

Also 2,2-dimethylbutane, and 2,3-dimethylbutane
There are five structural isomers with the formula C6H14. Two of these isomers are 2,2-dimethylbutane and 2,3-dimethylbutane.
2,2-dimethylbutane, also known as neohexane, is an organic compound with the formula C6H14 or (H3C-)3-C-CH2-CH3. It is the most compact and branched of the hexane isomers and the only one with a quaternary carbon and a butane (C4) backbone. It was discovered in 1872 by Butlerov's student V. Goryainov through the cross-coupling of zinc ethyl with tert-butyl iodide. It can be synthesized by the hydroisomerisation of 2,3-dimethylbutane using an acid catalyst or by isomerization of n-pentane in the presence of a catalyst containing palladium, platinum, rhodium, or rhenium on a matrix.
2,3-dimethylbutane, on the other hand, is an isomer of hexane with the formula (CH3)2CHCH(CH3)2. It is a colorless liquid with a boiling point of 57.9 °C. It can be converted to 2,2-dimethylbutane through hydroisomerisation, as mentioned earlier.
Supreme Court's Power: Amending the Constitution
You may want to see also
Explore related products
$42.23 $49.99

They are compounds with the same formula but different atom connections
The formula C6H14 refers to a group of compounds known as isomers. Isomers are molecules with the same molecular formula but different structures or arrangements of atoms. In other words, they have the same number of atoms of each element but are structurally distinct. For example, two bracelets made of five red and five green beads can be rearranged in many isomeric forms, depending on the order of the colours. Each bracelet has the same number of beads, but each variation will look different.
There are two main types of isomers: constitutional isomers and stereoisomers. Constitutional isomers, also known as structural isomers, are molecules with different connectivity. They have the same parts, but those parts are attached differently. For instance, butane (C4H10) and isobutane are constitutional isomers. Butane has its four carbon atoms bonded in a continuous chain, while isobutane has a branched structure.
Stereoisomers, on the other hand, have the same connectivity but differ in the orientation of their parts in space. They can be further classified into two types: enantiomers and diastereomers. Enantiomers are like a pair of hands – they are mirror images of each other but cannot be superimposed. Diastereomers, on the other hand, are all the other types of stereoisomers that are not enantiomers.
Conformational isomers, also known as conformers, are a type of stereoisomer that differs from one another by their rotation around a single bond. Single bonds, unlike double and triple bonds, are not "locked" in their orientation and can rotate freely.
There are five structural isomers for the formula C6H14.
The Constitution: Military and Federal Government Explained
You may want to see also

The number of possible isomers increases with carbon atoms
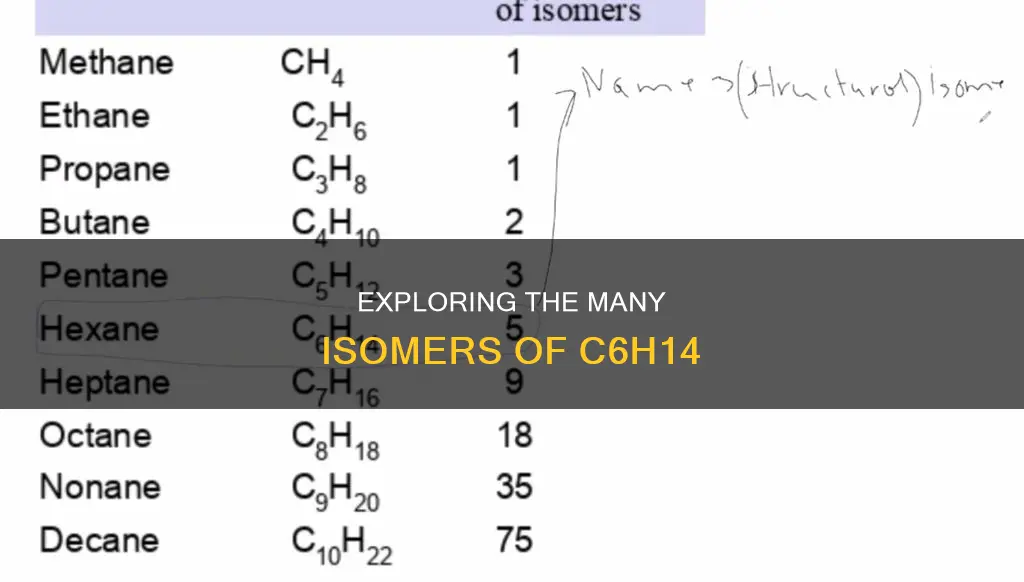
The molecular formula C6H14 has five possible structural isomers. The number of possible isomers increases as the number of carbon atoms increases. This is due to carbon's unique ability to form four bonds with other atoms, allowing for the formation of long chains or branched structures. With each additional carbon atom, the number of potential configurations and connectivities increases significantly.
For example, butane (C4H10) has only two isomers due to the limited arrangement of its four carbon atoms. In contrast, decane (C10H22), with its ten carbon atoms, has 75 possible isomers. The increase in carbon atoms allows for more branching and different structural arrangements, leading to a higher number of isomers.
The concept of isomers is based on the understanding that compounds with different physical properties can have the same elemental composition and molecular weight. Constitutional isomers, in particular, have the same molecular formula but differ in the structural arrangement of their atoms. This structural diversity increases exponentially with each additional carbon atom, showcasing the vast diversity of organic structures.
The relationship between the number of carbon atoms and the number of possible isomers is evident in the example of butane and decane. The increase in carbon atoms from four to ten results in a significant jump in the number of isomers, from two to 75. This highlights how the structural complexity and diversity of alkanes grow as the number of carbon atoms increases, leading to a higher number of potential isomers.
The Politician's Promise: Oath to the Constitution
You may want to see also
Frequently asked questions
There are 5 constitutional isomers.
Constitutional isomers are compounds that have the same molecular formula but different connections among their atoms.
The isomers are hexane, 2-methylpentane, 3-methylpentane, 2,2-dimethylbutane, and 2,3-dimethylbutane.
It represents an alkane, a hydrocarbon with only single bonds.
Yes, there are also structural isomers for this molecule.