There are five constitutional isomers with the molecular formula C6H14. One of these isomers is Isomer D, or 2,3-dimethylbutane, which has two methyl groups attached to a butane chain. This isomer has three unique sets of hydrogens available for chlorination, and it yields three monochlorination products when treated with chlorine at 300°C.
| Characteristics | Values |
|---|---|
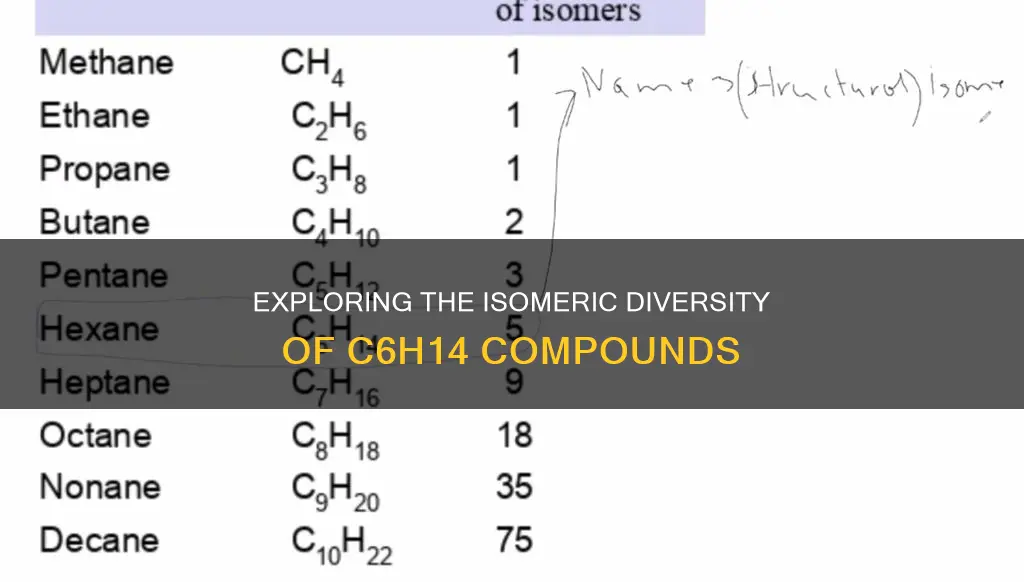
| Number of constitutional isomers | 5 |
| Number of possible structural isomers | 3, 6, 4, 5 |
| Example of an isomer | 2,3-dimethylbutane |
Explore related products
What You'll Learn
- There are five constitutional isomers with the molecular formula C6H14
- One of the isomers is 2,3-dimethylbutane
- It has three unique sets of hydrogens
- The number of monochlorination products corresponds to the number of unique sets of equivalent hydrogens
- The number of possible structural isomers is 3, 6, 4, or 5

There are five constitutional isomers with the molecular formula C6H14
The five constitutional isomers of C6H14 are likely to include 2,3-dimethylbutane, which has two methyl groups attached to a butane chain. This particular isomer has three unique sets of hydrogens available for chlorination, resulting in three monochlorination products. The number of monochlorination products is indicative of the number of unique sets of equivalent hydrogens available for substitution.
The other four isomers of C6H14 are not as well-defined in the sources provided, but it is likely that they have similar structural variations, resulting in different arrangements of carbon and hydrogen atoms. These variations lead to different physical properties, despite the compounds having the same elemental composition and molecular weight.
The number of possible isomers increases as the number of carbon atoms increases. This is because a higher number of carbon atoms allows for more structural variations, resulting in a greater number of possible isomers. In the case of C6H14, there are five possible structural isomers, showcasing the complex nature of organic chemistry and the diverse arrangements of atoms that can result in unique compounds.
Mayflower Compact's Influence on US Constitution
You may want to see also

One of the isomers is 2,3-dimethylbutane
There are five structural isomers with the molecular formula C6H14. One of the isomers is 2,3-dimethylbutane, which can undergo hydrogenolysis over supported catalysts of ruthenium, nickel, cobalt, and iron to form smaller hydrocarbons, primarily methane. The chemical synthesis and structural analysis of a novel compound derived from hexaoxocyclohexane and 2,3-dimethylbutane are discussed in a paper by EV Tretyakov and AO Tkacheva (2014). Another paper by P Rouge et al. (2019) focuses on the development of a selective catalyst system for the dehydrogenation of 2,3-dimethylbutane, highlighting the stability and efficiency of the catalyst.
Now, let's delve deeper into the properties and applications of 2,3-dimethylbutane. This isomer has a unique structure where two methyl groups are attached to the second and third carbon atoms of a butane chain. Its molecular formula is C6H14, indicating that it contains six carbon atoms and fourteen hydrogen atoms. The presence of multiple methyl groups contributes to its chemical reactivity and physical properties.
2,3-Dimethylbutane belongs to the family of alkanes, which are known for their carbon-carbon single bonds and carbon-hydrogen bonds. Alkanes are typically non-polar and hydrophobic, preferring other non-polar molecules in their surroundings. This isomer is a volatile liquid at room temperature, with a boiling point of around 50°C. It has a mild odour and is colourless.
In terms of reactivity, 2,3-dimethylbutane can undergo various chemical reactions. As mentioned earlier, it can be dehydrogenated using specific catalysts, leading to the formation of smaller hydrocarbons. This process involves the removal of hydrogen from the molecule, resulting in the breakage of carbon-carbon bonds and the creation of new double or triple bonds.
Additionally, 2,3-dimethylbutane can undergo hydrogenolysis, which is the reverse process of hydrogenation. In this reaction, the molecule interacts with hydrogen molecules, adding hydrogen atoms across double or triple bonds. This reaction can be catalysed by various transition metals, as mentioned earlier. The products of hydrogenolysis are often smaller hydrocarbons, demonstrating the complex reactivity of this isomer.
Overall, 2,3-dimethylbutane serves as a versatile chemical compound with applications in synthetic chemistry and catalysis. Its unique structure and reactivity profile make it a subject of academic research, as evidenced by the papers referenced earlier. By understanding its properties and behaviour, scientists can design novel catalysts and explore new chemical transformations.
Louisiana Purchase: Unconstitutional Expansion of America
You may want to see also

It has three unique sets of hydrogens
There are five constitutional isomers with the molecular formula C6H14. One of these isomers, known as Isomer D, has three unique sets of hydrogen atoms. This isomer has the IUPAC name 2,3-dimethylbutane.
The molecule 2,3-dimethylbutane has three types of hydrogen atoms. These are:
- Hydrogen atoms on the two methyl groups at the ends of the molecule. These are equivalent to each other.
- Hydrogen atoms on the two methyl groups attached to the second and third carbon atoms. These are equivalent to each other.
- Hydrogen atoms on the second and third carbon atoms. These are equivalent to each other.
The presence of three unique sets of hydrogens in 2,3-dimethylbutane allows for different positions of chlorination, leading to multiple unique monochlorination products. This relationship between the structure of an isomer and the products formed during chlorination is well-documented in organic chemistry.
In summary, the constitutional isomer of C6H14 with three unique sets of hydrogens is 2,3-dimethylbutane. This isomer has three distinct types of hydrogen atoms, which play a crucial role in determining the isomer's reactivity and unique monochlorination products.
The District of Columbia: A Constitutional Conundrum
You may want to see also
Explore related products

The number of monochlorination products corresponds to the number of unique sets of equivalent hydrogens
There are five constitutional isomers with the molecular formula C6H14. The number of monochlorination products that can be formed from these isomers depends on the number of unique sets of equivalent hydrogens available for substitution.
Monochlorination involves replacing hydrogen atoms with chlorine atoms in the original molecule. The number of possible monochlorination products is equal to the number of unique sets of equivalent hydrogens in the original molecule. This is because each unique set of hydrogens can be replaced by chlorine to form a distinct monochlorination product.
For example, consider the isomer 2,3-dimethylbutane, which has the molecular formula C6H14. This isomer has three unique sets of hydrogen atoms: those on the two methyl groups at the ends, those on the two methyl groups attached to the second and third carbon atoms, and those on the secondary carbon atom. Therefore, it can form three distinct monochlorination products.
The number of monochlorination products can also be influenced by other factors, such as the stability of the resulting alkyl radicals and the symmetry of the molecule. For example, a molecule with C2 symmetry will have half the number of individual monochlorination products compared to a molecule without such symmetry.
In summary, the number of monochlorination products that can be formed from a molecule with the formula C6H14 corresponds to the number of unique sets of equivalent hydrogens available for substitution, and this number can be influenced by various factors such as the stability of the resulting radicals and the symmetry of the molecule.
Thomas Jefferson's Influence on the US Constitution
You may want to see also

The number of possible structural isomers is 3, 6, 4, or 5
The molecular formula $C_{ 6 }H_{ 14 }$ represents hexane, a six-carbon compound and a member of the homologous series of alkanes. This compound has several structural isomers, which are compounds with the same number and type of atoms but differ in their structural formula or spatial arrangement.
The number of possible structural isomers for $C_{ 6 }H_{ 14 }$ is a topic of discussion, with some sources stating that there are 3, 6, 4, or 5 isomers, while others confirm only the latter three options.
Considering the different types of carbon atoms involved, we can identify four possible isomer structures. Firstly, there is only one isomer with only primary and secondary carbon atoms, named n-hexane or hexane. Secondly, there are two isomers with one tertiary carbon atom: isopentane or 2-methylpentane, and 3-methylpentane. Thirdly, there is one isomer with two tertiary carbon atoms, known as 2,3-dimethylbutane. Lastly, there is one isomer with a quaternary carbon atom, neo-hexane or 2,2-dimethylbutane. This gives us a total of five structural isomers for $C_{ 6 }H_{ 14 }$.
However, it's important to recognize that some sources simply state the possible answers as 3, 6, 4, or 5, without providing further clarification or a definitive answer.
Federal Record Status: What Counts and Why?
You may want to see also
Frequently asked questions
There are five constitutional isomers with the molecular formula C6H14.
One such isomer is Isomer D, also known as 2,3-dimethylbutane, which yields three monochlorination products when treated with chlorine at 300°C.
To determine the structure of Isomer D, we need to consider the arrangement of carbon and hydrogen atoms that can result in three monochlorination products. This isomer has three types of hydrogen atoms, leading to three distinct monochlorination outcomes.
Compounds with the same elemental composition and molecular weight but different physical properties are known as isomers. For example, monochlorination of compound A results in two constitutional isomers, while compound B forms four constitutional isomers.