There are three constitutional isomers with the molecular formula C5H12, also known as pentanes. These isomers are identified as isopentane (isomer A), pentane (isomer B), and neopentane (isomer C) and they differ in terms of their branching and symmetry. The number of monochlorination products they yield when reacted with chlorine at 300°C helps distinguish them.
| Characteristics | Values |
|---|---|
| Number of constitutional isomers | 3 |
| Isomer A | isopentane, yielding four products |
| Isomer B | pentane, yielding three products |
| Isomer C | neopentane, yielding a single product |
Explore related products
What You'll Learn

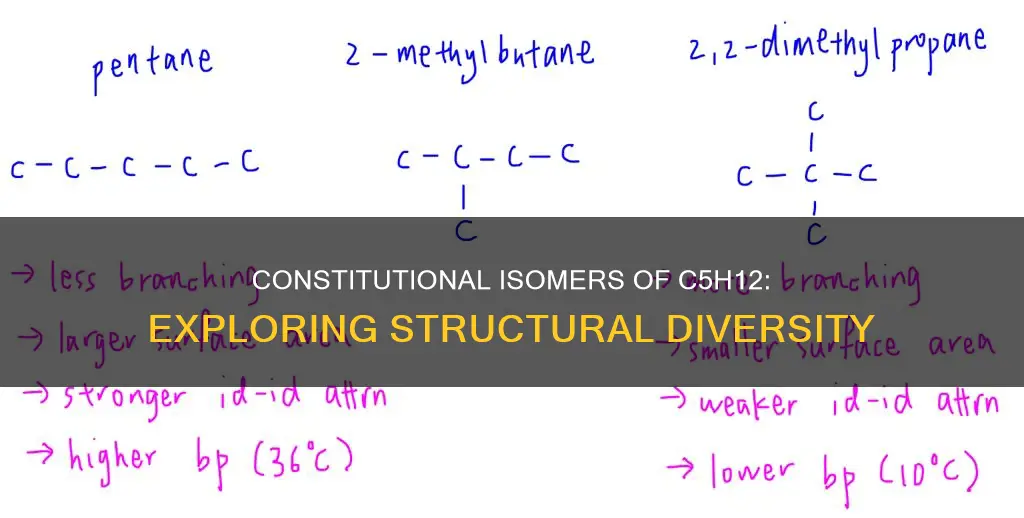
There are three constitutional isomers of C5H12
Isomer A, or isopentane, yields four monochlorination products and has the formula (CH3)2CHCH2CH3. This isomer has a branched structure, allowing for different types of hydrogens that can be replaced by chlorine to give unique products. Isomer B, or pentane, yields three products and has a linear structure, with the formula H3CCH2CH(CH3)2. Finally, Isomer C, or neopentane, yields only one product and has a symmetrical structure, with the formula (CH3)4C.
The difference in the number of monochlorination products is due to the varying structures of the isomers. Isomer A's branched structure allows for the most variation in the replacement of hydrogen atoms with chlorine atoms, resulting in the highest number of unique products. Isomer B's linear structure falls in between Isomer A and Isomer C in terms of complexity and symmetry, yielding three products. Isomer C's symmetrical structure means that all hydrogen atoms are equivalent, resulting in only one unique product.
The three isomers of C5H12—isopentane, pentane, and neopentane—can be distinguished by their structural differences, reactivity, and the variety of products they yield during chlorination reactions.
Who Really Wrote the US Constitution Preamble?
You may want to see also

Isopentane, Pentane, and Neopentane
There are three possible constitutional isomers for C5H12: n-pentane, isopentane (methylbutane), and neopentane (dimethylpropane). These isomers differ in their molecular structure and physical properties, such as boiling and melting points. Let's explore the characteristics of isopentane, pentane, and neopentane in more detail.
Isopentane
Isopentane, also known as methylbutane, is one of the isomers of C5H12. It has a lower boiling point compared to n-pentane and neopentane, making it the most volatile liquid alkane at room temperature. Isopentane's boiling point is approximately 27.7 °C, and its melting point is -159.9 °C. It is produced through acid-catalyzed isomerization and is used in the production of high-octane fuels. Isopentane is also employed as a working medium in geothermal power stations and organic Rankine cycles due to its low boiling point, low cost, and safety profile.
Pentane
Pentane, specifically referring to the n-pentane isomer, is a linear or straight-chain isomer of C5H12. It has a higher boiling point than isopentane, with a value of 36.0 °C, and a melting point of -129.8 °C. Pentane was discovered in 1862 by Carl Schorlemmer during his analysis of pyrolysis products of cannel coal. It is produced by fractional distillation of petroleum and is used in various applications, including as a solvent in laboratories and in the production of polystyrene foam. Pentane is relatively unreactive at standard room temperature and conditions but can be oxidized with sufficient activation energy, such as an open flame.
Neopentane
Neopentane, also known as dimethylpropane, is the most heavily branched isomer of C5H12. It has the lowest boiling point among the three isomers, at 9.5 °C, and is the only one that exists as a gas at room temperature and atmospheric pressure. On the other hand, neopentane exhibits an anomalously high melting point of -16.6 °C, which has been attributed to the tetrahedral symmetry of its molecules, allowing for closer packing in solid form. Neopentane's full tetrahedral symmetry results in all chemically equivalent protons, leading to a distinct NMR chemical shift when dissolved in carbon tetrachloride.
Carolina's Constitution: Still Relevant Today?
You may want to see also

They have different structures and symmetries
There are three isomers of C5H12 or pentane: n-pentane, methylbutane (or 2-methylbutane), and neopentane (or 2,2-dimethylpropane). These isomers have the same molecular formula but different structural formulas or two-dimensional representations.
Let's take a closer look at each isomer's structure and symmetry:
N-pentane, or normal-pentane, is the straight-chain isomer of C5H12. It has a simple linear structure with five carbon atoms bonded in a straight chain, and each carbon atom is bonded to two hydrogen atoms. The symmetry in n-pentane is evident in its straight chain structure, where all carbon and hydrogen atoms are arranged symmetrically along the carbon backbone.
Methylbutane, or 2-methylbutane, is one of the branched-chain isomers of C5H12. In this isomer, one of the carbon atoms in the chain has a methyl group (CH3) attached, resulting in a branch-like structure. The symmetry in methylbutane is disrupted by the presence of the methyl group, which breaks the uniformity of the carbon chain.
Neopentane, or 2,2-dimethylpropane, is another branched-chain isomer of C5H12. In this isomer, the central carbon atom is bonded to four methyl groups (CH3). This structure results in a very compact and symmetrical molecule. The symmetry in neopentane is evident as the four methyl groups are evenly distributed around the central carbon atom, forming a tetrahedral shape.
While n-pentane exhibits symmetry in its linear structure, methylbutane and neopentane display different types of symmetry due to their branched structures. The arrangement of atoms in these isomers affects their physical and chemical properties, such as boiling points and reactivity.
In summary, the three isomers of C5H12, n-pentane, methylbutane, and neopentane, exhibit different structures and symmetries due to the varying arrangements of carbon and hydrogen atoms. These structural differences play a crucial role in understanding the unique characteristics of each isomer.
The US Constitution: A Global Impact Study
You may want to see also
Explore related products
$42.23 $49.99

This affects chlorination outcomes
C5H12, also known as pentane, has three isomers: n-pentane, methylbutane, and neopentane. Now, onto how this affects chlorination outcomes.
Chlorination is a popular method of water disinfection that has been used for many years. Chlorine is added to water to kill bacteria, viruses, and other microorganisms that cause diseases. The concentration of chlorine and the chlorine contact time are crucial factors in the disinfection process. Higher chlorine concentrations and longer contact times lead to more effective disinfection. However, chlorine can also be toxic to humans, acting as an irritant to the eyes, nasal passages, and respiratory system.
During water treatment, chlorine reacts with naturally occurring organic matter to form disinfection byproducts (DBPs), which can have negative health effects with long-term exposure. These DBPs include trihalomethanes, which are carcinogenic, and chlorophenols, which affect respiration and energy storage processes and cause taste and odour issues. To minimize these adverse effects, public water systems strive to maintain chlorine levels that balance effective disinfection with minimal taste and odour impacts.
In sewage treatment, chlorination can significantly reduce culturable bacterial cells. However, some bacteria can enter a viable but non-culturable (VBNC) state, maintaining membrane integrity and metabolic activities despite chlorine stress. This poses a public health concern as these VBNC bacteria may regain culturability and proliferate after the chlorine stress is removed. Additionally, resistant bacteria may survive chlorination and enter aquatic environments upon effluent discharge, particularly in seawater-based effluents.
Overall, the structural isomers of C5H12 impact chlorination outcomes by influencing the organic matter that reacts with chlorine during water treatment. The specific isomer present affects the formation of DBPs, including toxic and carcinogenic compounds, which ultimately influences the disinfection efficacy and potential health risks associated with chlorinated water.
Presidential Requirements: The Constitutional Guide
You may want to see also

The isomers have different numbers of monochlorination products
There are three constitutional isomers of C5H12: n-pentane, isopentane (2-methylbutane), and neopentane (2,2-dimethylpropane). These isomers differ in the structure and arrangement of their carbon backbone and the resulting shape of the molecule. The number of carbon atoms and the presence of carbon-carbon bonds determine the number of monochlorination products possible for each isomer. Monochlorination refers to the replacement of a hydrogen atom in the molecule with a chlorine atom.
Starting with n-pentane, which has the formula CH3-CH2-CH2-CH2-CH3, there are five different monochlorinated products that can be formed. This is because there are five hydrogen atoms on the terminal carbon atoms (at both ends of the molecule) that can be substituted with chlorine. Each terminal carbon has three hydrogen atoms attached, and the chlorine atom can replace any of them, leading to five possible monochlorinated derivatives.
For isopentane (2-methylbutane), with the formula CH3)3C-CH2-CH3, there are also five monochlorinated products possible. However, unlike n-pentane, not all monochlorinated derivatives are structurally distinct. There are two terminal carbon atoms in isopentane that each have three hydrogen atoms. Substitution of chlorine for any of these hydrogens leads to four possible monochlorinated products. Additionally, there is one hydrogen atom bonded to a carbon atom directly attached to the central carbon (the one with three methyl groups), which can also be replaced by chlorine, giving the fifth monochlorinated product.
Neopentane (2,2-dimethylpropane), with the formula CH3)2C=C(CH3)2, has a different story to tell. Here, we find only three monochlorinated products. The molecule has no terminal carbon atoms with hydrogen substituents, limiting the monochlorination possibilities. Instead, there are four hydrogen atoms attached to the two carbon atoms directly bonded to the central carbon. Substituting chlorine for any of these four hydrogens results in the three monochlorinated derivatives.
The variation in the number of monochlorination products among the isomers arises from the distinct arrangements of carbon atoms and the resulting exposure of hydrogen atoms. The number of terminal carbon atoms, the number of hydrogen atoms on these terminal carbons, and the accessibility of these hydrogen atoms to substitution all play a role in determining the possible monochlorinated derivatives for each isomer.
In conclusion, the three isomers of C5H12 exhibit different behaviors when subjected to monochlorination. n-Pentane and isopentane offer five monochlorinated products each, although not all of these structures are unique in the case of isopentane. On the other hand, neopentane yields only three monochlorinated derivatives due to its restricted structure. This example illustrates how the choice of isomer can influence the range of products obtained in a reaction, providing chemists with a tool to predict and control the outcome of synthetic processes.
Exploring Religious Freedom in Russia's Constitution
You may want to see also
Frequently asked questions
There are three constitutional isomers of C5H12.
The isomers are isopentane (or methylbutane), pentane, and neopentane (or dimethylpropane).
The isomers differ in structure, with isopentane being branched, pentane being linear, and neopentane being symmetrical.
The structural differences influence reactivity and product variety when treated with chlorine. For example, isopentane yields four monochlorination products, pentane yields three, and neopentane yields one.
Monochlorination products are formed when a compound is heated with chlorine, resulting in the substitution of hydrogen atoms with chlorine atoms. The number and type of products depend on the structure of the original compound.