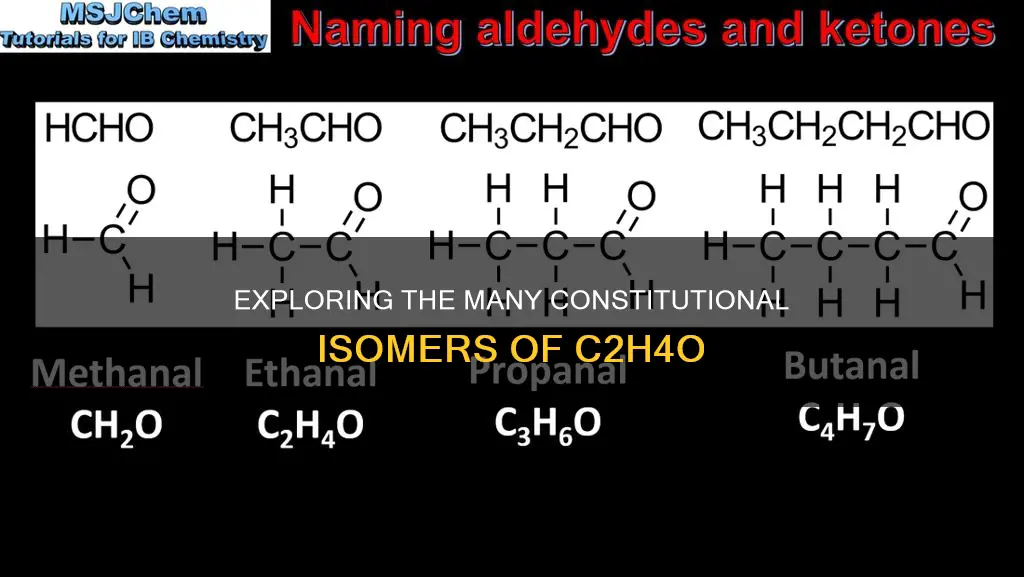
The molecular formula C2H4O can signify two constitutional isomers: ethanol and dimethyl ether. However, some sources state that there are three C2H4O isomers: acetaldehyde, ethylene oxide, and vinyl alcohol. These isomers have been studied in laboratory experiments and multi-dimensional quantum mechanical simulations to understand their formation and interactions in various states. The concept of constitutional isomers is prevalent in organic chemistry, where these isomers share the same molecular formula but differ in their atomic arrangements, influencing their physical and chemical characteristics.
| Characteristics | Values |
|---|---|
| Number of Constitutional Isomers | 2 or 3 |
| Names of Isomers | Ethanol, Dimethyl Ether, Acetaldehyde, Ethylene Oxide, Vinyl Alcohol |
| Bond-line Structure | Ethanol: C-C-O-H; Dimethyl Ether: C-O-C |
Explore related products
What You'll Learn
- There are two constitutional isomers of the formula C2H4O
- Constitutional isomers have the same molecular formula but different skeletal structures
- The two isomers of C2H4O are ethanol and dimethyl ether
- C2H4O can also form three isomers: acetaldehyde, ethylene oxide, and vinyl alcohol
- These isomers have been observed in interstellar ices

There are two constitutional isomers of the formula C2H4O
The concept of constitutional isomers is well-established in organic chemistry, where the arrangement of atoms and bonds can significantly affect the physical and chemical properties of a molecule. In the case of C2H4O, the oxygen atom forms two bonds with the neighboring carbon or hydrogen atoms, and the remaining electrons exist as lone pairs. This unique bonding configuration allows for the formation of two distinct constitutional isomers.
It is important to note that while there are two primary constitutional isomers of C2H4O, there are also three identified isomers: acetaldehyde (CH3CHO), ethylene oxide (c-C2H4O), and vinyl alcohol (CH2CHOH). These three isomers have been identified in laboratory experiments and simulations involving interstellar and cometary ices. The formation of these isomers through electronic energy transfer processes initiated by electrons in the track of MeV ion trajectories has been studied extensively.
The identification and understanding of constitutional isomers, such as those of C2H4O, are crucial in various scientific fields, especially astrochemistry. By studying the formation mechanisms of these isomers in extraterrestrial environments, scientists can gain insights into the astrochemical evolution of the interstellar medium and the origins of life. Additionally, the presence of certain isomers, such as ethylene oxide, in interstellar ices suggests that ethanol may serve as a critical precursor in these environments, further enhancing our understanding of the formation of complex organic molecules in space.
Private School Constitutional Compliance: What's the Law?
You may want to see also

Constitutional isomers have the same molecular formula but different skeletal structures
Constitutional isomers are compounds that have the same molecular formula but differ in their skeletal structures. They are also known as structural isomers. This means that constitutional isomers have the same types and numbers of atoms but differ in the way these atoms are connected or bonded together. These differences in connectivity lead to distinct atomic arrangements and bonding patterns, resulting in unique physical and chemical properties.
For example, ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3) are constitutional isomers of the molecular formula C2H4O. Ethanol has a straight-chain structure, while dimethyl ether has a branched structure. Their bond-line structures differ accordingly: Ethonal (C-C-O-H) and Dimethyl ether (C-O-C).
The number of constitutional isomers for a given molecular formula increases exponentially as the number of atoms in that formula increases. For instance, while there is only one possible isomer for CH4 (methane) and C2H6 (ethane), there are 355 possible isomers for dodecane (C12H26). This highlights the importance of understanding constitutional isomers and their unique characteristics.
Constitutional isomers can be further classified into skeletal isomers (or chain isomers) and positional isomers (or regioisomers). Skeletal isomers differ in the ordering of the skeleton of the molecule, resulting in different chain configurations. On the other hand, positional isomers have functional groups attached at different positions on the molecule, leading to distinct structural arrangements.
In summary, constitutional isomers share the same molecular formula but exhibit different skeletal structures due to variations in atomic connectivity and bonding patterns. These differences profoundly impact their physical and chemical properties. The study of constitutional isomers is essential in organic chemistry to understand and distinguish between compounds with similar formulas but unique characteristics.
Supreme Court: Beyond the Constitution
You may want to see also

The two isomers of C2H4O are ethanol and dimethyl ether
The molecular formula C2H4O can signify two constitutional isomers: ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3). Constitutional isomers, also known as structural isomers, are a type of isomer with the same molecular formula but a different skeletal structure due to the arrangement of atoms and bonds. In the case of ethanol and dimethyl ether, the difference lies in their chain configuration. Ethanol has a straight-chain structure, while dimethyl ether has a branched structure.
Ethanol and dimethyl ether have been detected in several low-, intermediate-, and high-mass star-forming regions, including Sgr B2, Orion, and W33A. The relative abundance ratios of ethanol to dimethyl ether vary in these regions, ranging from approximately 0.03 to 3.4. The synthesis of ethanol is favored over dimethyl ether in experimental branching ratios, with a ratio of 31 ± 11:1.
The bond-line structures of ethanol and dimethyl ether can be represented as follows:
Ethanol: C-C-O-H
Dimethyl ether: C-O-C
In these structures, the corners and ends represent carbon (C) atoms, the lines indicate the bonds, and the O's represent oxygen (O) atoms. Additionally, to represent lone pairs, add a line perpendicular to the oxygen atom in each diagram, indicating one lone pair.
While ethanol and dimethyl ether have the same molecular formula, their distinct structural arrangements lead to differences in their physical and chemical properties. This concept of constitutional isomers is well-established in organic chemistry, providing a foundation for understanding the diverse nature of chemical compounds.
The Constitution's Shadow: Black People's Constitutional Status
You may want to see also
Explore related products

C2H4O can also form three isomers: acetaldehyde, ethylene oxide, and vinyl alcohol
The molecular formula C2H4O can represent two different constitutional isomers: ethanol and dimethyl ether. However, C2H4O can also form three other isomers: acetaldehyde, ethylene oxide, and vinyl alcohol.
Laboratory studies have been conducted to understand the formation of these three C2H4O isomers in extraterrestrial ices via electronic energy transfer processes. These studies suggest that suprathermal oxygen atoms can add to the carbon-carbon π bond of an ethylene molecule to form an oxirene diradical and the cyclic ethylene oxide molecule. The oxirene diradical can then undergo a [1, 2]-H shift to form acetaldehyde. Both ethylene oxide and acetaldehyde isomers can be stabilized in the surrounding ice matrix.
The mechanism for the formation of these three C2H4O isomers has been analyzed using a 0-D homogeneous reactor kinetics simulation for ethylene oxidation. Kinetic models have also been used to explore the mechanism of formation of these isomers in the oxidation of ethylene.
Observations have revealed gaseous abundances of acetaldehyde and oxirane (another name for ethylene oxide) in high-mass star-forming regions. Furthermore, the presence of acetaldehyde and ethylene oxide has been suggested in the atmosphere of Titan, highlighting the importance of understanding their formation processes in astrochemistry.
A Future Without the US Constitution: Possibility or Pipe Dream?
You may want to see also

These isomers have been observed in interstellar ices
The molecular formula C2H4O can signify two constitutional isomers: ethanol and dimethyl ether. These two isomers differ in their chain configuration. Ethanol (CH3CH2OH) has a straight-chain structure, while dimethyl ether (CH3OCH3) has a branched structure.
There are three C2H4O isomers that have been observed in interstellar ices: acetaldehyde (ethanal), vinyl alcohol (ethenol), and oxirane (ethylene oxide). These isomers have been the subject of laboratory investigations to understand their formation in interstellar ices. Laboratory experiments have been conducted to unravel the synthetic routes to form these three isomers in extraterrestrial ices via electronic energy transfer processes. These processes are initiated by electrons in the track of MeV ion trajectories.
The results of electron irradiation on a 2:1 mixture of carbon dioxide (CO2) and ethylene (C2H4) suggest that suprathermal oxygen atoms can add to the carbon-carbon bond of an ethylene molecule to form an oxirene diradical. This oxirene diradical can then undergo a [1, 2]-H shift to form acetaldehyde. The abundances of these isomers are a factor of 2-600 higher than predicted by pure gas-phase models, indicating that solid-state processes play a significant role in their formation.
In addition to these three isomers, glycolaldehyde (HCOCH2OH) and methyl formate (HCOOCH3) have also been observed in interstellar ice analogs. Binary mixtures of methanol (CH3OH) and carbon monoxide (CO) ices were irradiated at 10 K with energetic electrons to mimic the energy transfer processes that occur in the trajectories of MeV cosmic-ray particles. The formation of glycolaldehyde was established through the appearance of new bands in the infrared spectrum.
The detection of these isomers in interstellar ices provides valuable insights into the present and past physiochemical conditions of molecular clouds and cores.
Executive Agencies: Independence vs Cabinet Control
You may want to see also
Frequently asked questions
There are two constitutional isomers of the formula C2H4O: ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3).
Ethanol has a straight-chain structure, whereas dimethyl ether has a branched structure.
The bond-line structure for ethanol is C-C-O-H, and for dimethyl ether, it is C-O-C.