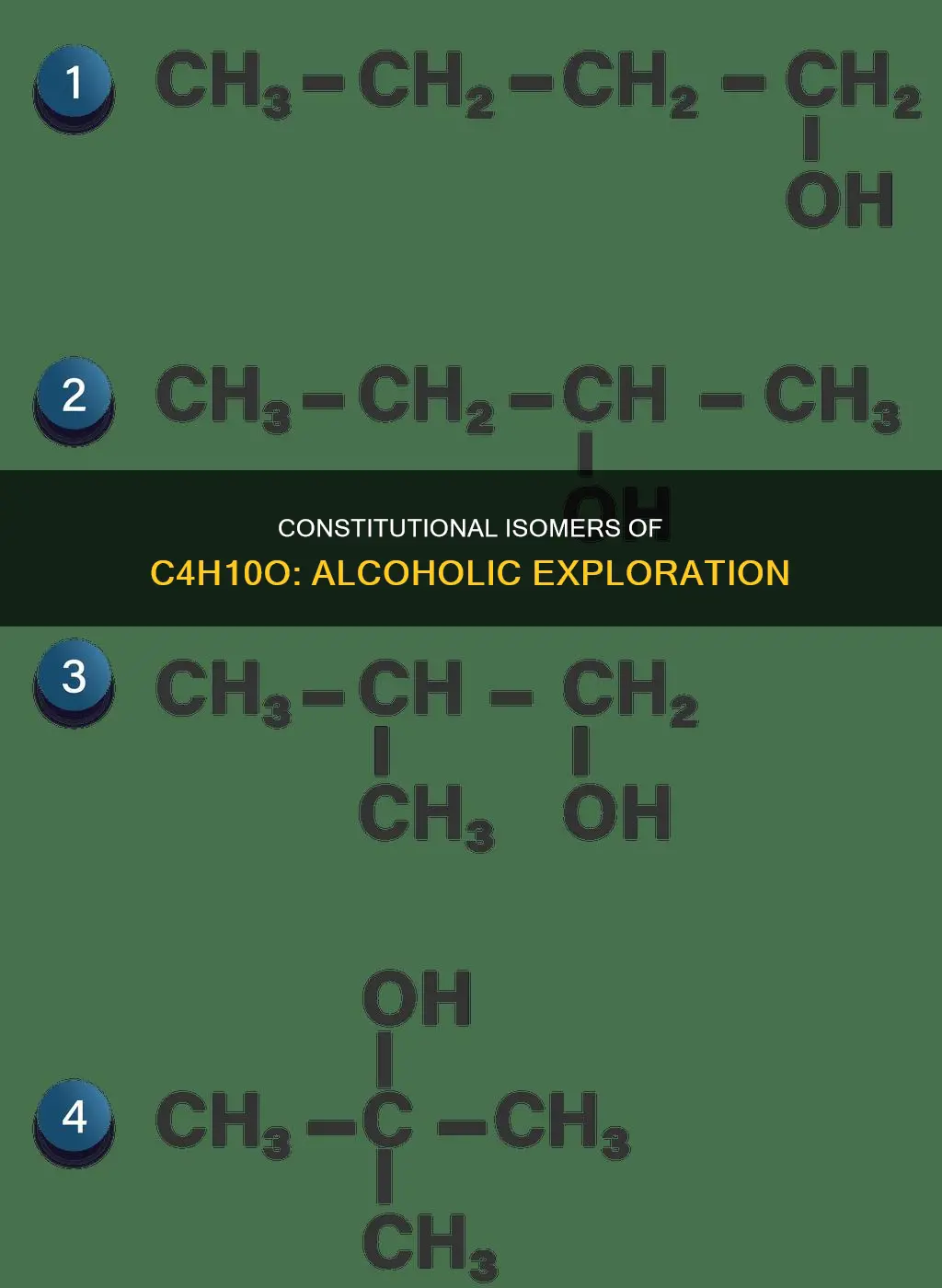
There are four constitutional isomers of C4H10O that contain alcohols. These isomers, which include 2-methyl-1-propanol, 1-methyl-2-propanol, 1-butanol, and 2-butanol, each have a unique structural arrangement despite sharing the same molecular formula.
| Characteristics | Values |
|---|---|
| Number of Carbon Atoms | 4 |
| Number of Hydrogen Atoms | 10 |
| Number of Oxygen Atoms | 1 |
| Possible Constitutional Isomers | 2-methyl-1-propanol, 1-methyl-2-propanol, 1-butanol, 2-butanol |
Explore related products
What You'll Learn
- methyl-1-propanol is an isomer with molecular formula C4H10O
- methyl-2-propanol is another possible constitutional isomer
- butanol is an alcohol with a straight chain of four carbon atoms
- butanol is another alcohol with the same molecular formula
- Each isomer has a unique structure, despite sharing the same molecular formula

2-methyl-1-propanol is an isomer with molecular formula C4H10O
The molecular formula C4H10O represents an alcohol, a type of organic compound. Alcohols with this molecular formula have four carbon atoms, ten hydrogen atoms, and one oxygen atom. These atoms can be arranged in different ways, leading to the formation of distinct isomers. Isomers are compounds that share the same molecular formula but exhibit different structural formulas. In the context of C4H10O, this means that the carbon and hydrogen atoms can be arranged in various sequences, resulting in unique isomers.
One such isomer is 2-methyl-1-propanol, also known as isobutanol. This isomer is characterized by one carbon atom bonded to three hydrogen atoms and an -OH group. The same carbon atom also forms bonds with two additional carbon atoms and one more hydrogen atom. This specific arrangement of atoms distinguishes 2-methyl-1-propanol from other isomers of C4H10O.
The existence of isomers, such as 2-methyl-1-propanol, underscores the concept of isomerism in organic chemistry. Isomerism highlights the fact that compounds with identical molecular formulas can exhibit distinct structural arrangements. This phenomenon is not limited to C4H10O but is observed across various organic compounds, showcasing the diverse nature of chemical structures.
The molecular formula C4H10O represents a family of isomers, including not only 2-methyl-1-propanol but also other isomers like 1-methyl-2-propanol, 1-butanol, and 2-butanol. Each of these isomers has a unique structural configuration, emphasizing the variability within this group of compounds. This diversity in structure leads to differences in physical and chemical properties, influencing their behavior and applications in various contexts.
The isomer 2-methyl-1-propanol, or isobutanol, finds applications in various industries. For example, it is used as a solvent, either directly or through its esters. It is also a component of some biofuels, showcasing its versatility and importance in energy-related applications. The exploration and understanding of isomers, such as 2-methyl-1-propanol, contribute to our ability to harness their unique properties for specific purposes.
Senate's Core Duties: Understanding the Four Responsibilities
You may want to see also

1-methyl-2-propanol is another possible constitutional isomer
The molecular formula C4H10O represents an alcohol with four carbon atoms, ten hydrogen atoms, and one oxygen atom. One of its possible constitutional isomers is 1-methyl-2-propanol, also known as isobutanol. This isomer has a carbon atom connected to three hydrogen atoms and a hydroxyl (-OH) group. The carbon atom also forms bonds with two other carbon atoms and one hydrogen atom.
Isobutanol is a colourless, flammable liquid with a distinct odour. It is mainly used as a solvent, either in its pure form or as its esters. It is also a byproduct of grain fermentation and can be found in trace amounts in many alcoholic beverages. Isobutanol's structure was discovered in 1867 by Emil Erlenmeyer and Vladimir Markovnikov, who determined it by proving its oxidation product.
Isobutanol is often used interchangeably with 1-butanol, and they share similar applications. They are commonly employed as varnishes and precursors to esters, which are valuable solvents. For example, isobutyl acetate is an ester derived from isobutanol. Isobutyl esters of phthalic, adipic, and related dicarboxylic acids are frequently used as plasticizers.
Additionally, isobutanol is a component of some biofuels. It can be produced through the Guerbet condensation reaction of propanol and methanol. It can also be synthesised biologically by genetically modifying organisms such as E. coli to produce C4 alcohols from glucose.
Net Neutrality: A Constitutional Right or Wrong?
You may want to see also

1-butanol is an alcohol with a straight chain of four carbon atoms
The molecular formula of the alcohol is C4H10O, which means there are four carbon atoms, ten hydrogen atoms, and one oxygen atom in the molecule. One of the constitutional isomers of C4H10O is 1-butanol, also known as butan-1-ol or n-butanol. It is a primary alcohol with the chemical formula C4H9OH and a linear structure. It is a straight-chain primary alcohol with a butyl or isobutyl group linked to a hydroxyl group.
The unmodified term butanol typically refers to the straight-chain isomer with the alcohol functional group at the terminal carbon, which is also known as 1-butanol. It is one of the butanols, which are normally present in fusel alcohol. Butan-1-ol occurs naturally as a result of carbohydrate fermentation in several alcoholic beverages, including beer, grape brandies, wine, and whiskey. It has also been found in the volatiles of hops, jackfruit, heat-treated milk, musk melon, cheese, southern pea seed, and cooked rice.
1-Butanol is formed during the deep frying of corn oil, cottonseed oil, trilinolein, and triolein. It is a natural component of many alcoholic drinks, albeit in low and variable concentrations. It is used as an ingredient in processed and artificial flavorings and for the extraction of lipid-free protein from egg yolk. It is also used in a wide range of consumer products, such as perfumes, butter, cream, fruit, rum, ice cream, candy, baked goods, and cordials.
Constituting 85% of its use, 1-butanol is mainly used in the production of varnishes. It is also used as a solvent for a variety of chemical and textile processes, in organic synthesis, and as a chemical intermediate. It is a petrochemical derived from propylene.
Executive Orders: Constitutional Validity Explained
You may want to see also
Explore related products

2-butanol is another alcohol with the same molecular formula
There are four constitutional isomers of the molecule C4H10O that contain an alcohol functional group. This means that there are four different ways to arrange the atoms to form a compound with the formula C4H10O, where one of the hydrogen atoms is replaced by a hydroxyl (OH) group to create an alcohol. One of these isomers is 2-butanol, which can be formed by attaching the hydroxyl group to the second carbon atom in the butane chain.
The structure of 2-butanol is represented as CH3CHOHCH2CH3, where the OH group is bonded to the second carbon atom. It is an alcohol because it has the characteristic hydroxyl group (-OH) attached to a carbon atom. This carbon atom is usually attached to at least one hydrogen atom as well, but in this case, it is bonded to two other carbon atoms and a hydroxyl group. This carbon atom with the attached hydroxyl group is often referred to as the "alcohol carbon".
2-Butanol is a secondary alcohol, which means that the carbon atom with the OH group attached has two other carbon atoms bonded to it as well. This is in contrast to primary alcohols, where the carbon with the OH group has only one other carbon attached, and tertiary alcohols, where the carbon with the OH group is bonded to three other carbons. The classification of alcohols as primary, secondary, or tertiary is important because it influences their chemical reactivity and physical properties.
The presence of the hydroxyl group in 2-butanol gives it similar chemical properties to other alcohols. It can undergo reactions typical of alcohols, such as dehydration to form alkenes, substitution reactions to form ethers, and oxidation to form aldehydes or ketones. These reactions are characteristic of the functional group and are seen across all alcohols, regardless of their specific structure.
So, in summary, 2-butanol is one of the constitutional isomers of C4H10O that contains an alcohol group. Its structure and classification as a secondary alcohol give it unique properties and reactivity patterns within the family of alcohols. Understanding the isomers of a given molecular formula helps us predict and explain the behavior of these compounds in various chemical reactions and applications.
Opponents of the Constitution: The Anti-Federalists' Fight
You may want to see also

Each isomer has a unique structure, despite sharing the same molecular formula
Isomers are compounds that share the same molecular formula but have distinct structures and arrangements of atoms. They can be structural or stereoisomers. Structural isomers, or constitutional isomers, have differing bond connections between their atoms, whereas stereoisomers have the same bonds but differ in the relative positions of their atoms.
For example, 1-propanol and 2-propanol are isomers with the molecular formula C3H8O. They are both alcohols derived from propane, with a chain of three carbon atoms connected by single bonds. However, the hydroxyl group is bound to different carbons: at one end of the chain for 1-propanol, and in the middle for 2-propanol. This variation in the structural formula, despite the shared molecular formula, exemplifies the concept of isomerism.
The molecular formula of the alcohol C4H10O indicates four carbon atoms, ten hydrogen atoms, and one oxygen atom. Several constitutional isomers of alcohols with this molecular formula exist, including 2-methyl-1-propanol, 1-methyl-2-propanol, 1-butanol, and 2-butanol. Each of these isomers has a unique structural arrangement, or connectivity of atoms, despite sharing the same molecular formula.
The diversity of these isomers highlights the concept of isomerism in organic compounds. The number of possible isomers increases with the number of carbon atoms. For instance, dodecane (C12H26) has 355 possible isomers. Isomers may exhibit very different physical and chemical properties, such as boiling point, melting point, and reactivity, despite their shared molecular formula.
The Supreme Court's Role: Interpreting the Constitution
You may want to see also
Frequently asked questions
Constitutional isomers are compounds with the same molecular formula but different structural formulas. They have the same number of atoms but differ in how these atoms are connected.
There are four constitutional isomers of alcohols with the molecular formula C4H10O: 2-methyl-1-propanol, 1-methyl-2-propanol, 1-butanol, and 2-butanol.
2-butanol has a straight chain of four carbon atoms, with two of them connected to each other and to an -OH group.
In 2-methyl-1-propanol, one carbon atom is connected to three hydrogen atoms and an -OH group. This carbon atom also forms bonds with two additional carbon atoms and one hydrogen atom.