Dibromoethene, also known as ethylene dibromide, is an organic compound with the molecular formula C₂H₂Br₂. It has three structural isomers: 1,1-dibromoethene, (E)-1,2-dibromoethene, and (Z)-1,2-dibromoethene. The number and variety of isomers of dibromoethene arise from the different arrangements of bromine atoms relative to the carbon-carbon double bond, resulting in distinct molecular structures and properties. Understanding the isomeric forms of dibromoethene is essential in fields such as organic chemistry and chemical engineering, providing insights into the behaviour and reactivity of this compound.
| Characteristics | Values |
|---|---|
| Number of constitutional isomers | 3 |
| Molecular formula | C2H2Br2 |
| Names of isomers | (E)-1,2-Dibromoethene, *<co: 1,2>(Z)-1,2-Dibromoethene</co: 1,2>, *<co: 0,1,2>1,1-Dibromoethene</co: 0,1,2> |
| Characteristics of (E)-1,2-Dibromoethene | Two bromine atoms on opposite sides of the double bond, no net dipole moment |
| Characteristics of (Z)-1,2-Dibromoethene | Two bromine atoms on the same side of the double bond, net dipole moment |
| Characteristics of 1,1-Dibromoethene | Two bromine atoms attached to the same carbon atom, unequal charge distribution, net dipole moment |
Explore related products
What You'll Learn
- There are three isomers of dibromoethene: (E)-1,2-Dibromoethene, (Z)-1,2-Dibromoethene, and 1,1-Dibromoethene
- The two bromine atoms in 1,2-Dibromoethene are on opposite sides of the double bond
- In 1,1-Dibromoethene, the bromine atoms are on the same side of the double bond
- The C-Br bond in 1,1-dibromoethene is polar
- The two bromine atoms in (Z)-1,2-Dibromoethene are on the same side of the double bond

There are three isomers of dibromoethene: (E)-1,2-Dibromoethene, (Z)-1,2-Dibromoethene, and 1,1-Dibromoethene
There are three isomers of dibromoethene:
E)-1,2-Dibromoethene
This isomer is also known as trans-1,2-dibromoethene or simply 1,2-dibromoethene. It is a dihalogenated unsaturated compound with one bromine atom on each of the two carbon atoms. The "E" in its name stands for "entgegen", which is German for "against" or "opposite". This prefix is used to denote that the compound has a trans configuration, meaning that the bromine atoms are on opposite sides of the double bond.
Z)-1,2-Dibromoethene
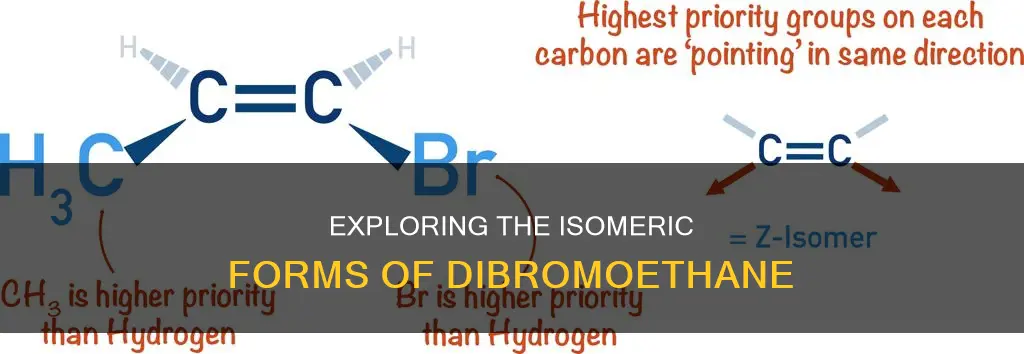
This isomer is also known as cis-1,2-dibromoethene. Like its counterpart, it is also a dihalogenated unsaturated compound with one bromine on each carbon atom. The "Z" in its name stands for "zusammen", which means "together" in German. This prefix indicates that the compound has a cis configuration, where the bromine atoms are on the same side of the double bond.
1,1-Dibromoethene
In this isomer, both bromine atoms are bonded to the same carbon atom, making it a geminal dibromide. This isomer has a different molecular structure from the other two isomers, which affects its chemical properties and behaviour.
It's important to note that all three isomers of dibromoethene are distinct chemical compounds with their own unique properties, despite sharing the same molecular formula. The position of the bromine atoms on the carbon chain influences their physical and chemical characteristics, reactivity, and behaviour in various reactions.
Albany Plan: A Constitution Forerunner
You may want to see also

The two bromine atoms in 1,2-Dibromoethene are on opposite sides of the double bond
Dibromoethene, or C2H2Br2, has three possible isomers, which are different molecular configurations with the same chemical formula. The arrangement of bromine atoms in relation to the carbon-carbon double bond determines whether the molecule has a net dipole moment or not. The two bromine atoms can be on the same side of the double bond, as in 1,1-dibromoethene, or on opposite sides, as in 1,2-dibromoethene. This is the isomer in which the two bromine atoms are on opposite sides of the double bond.
In 1,2-dibromoethene, the two bromine atoms are positioned on opposite sides of the double bond, which distinguishes it from the other isomers. This configuration results in the dipoles from the C-Br bonds opposing each other, leading to a molecule with no net dipole moment. This is in contrast to the other isomers, where the dipoles from the C-Br bonds add together, resulting in a net dipole moment.
The specific arrangement of atoms in 1,2-dibromoethene, with the bromine atoms on opposite sides of the double bond, is often referred to as the "trans" configuration. This term describes the spatial arrangement of atoms across a double bond, with the bromine atoms on opposite sides or "trans" to each other. This is in contrast to a "cis" configuration, where similar atoms or groups are positioned on the same side of the double bond.
The formation of 1,2-dibromoethene through a reaction between bromine and an alkene typically involves a mechanism called "anti" addition. In this process, the alkene attacks the bromine molecule, forming a bromonium ion. Subsequently, the backside of this ion is attacked by a bromide ion, resulting in the addition of bromine atoms to opposite sides of the original alkene. This "anti" addition is a key step in generating the isomer 1,2-dibromoethene, where the bromine atoms are indeed on opposite sides of the double bond.
The understanding of the spatial arrangement of atoms in 1,2-dibromoethene, particularly the positioning of bromine atoms on opposite sides of the double bond, is crucial in fields like organic chemistry and chemical research. It provides insights into the molecule's properties, reactivity, and potential applications. Additionally, the knowledge of this specific isomer contributes to the broader understanding of dibromoethene isomers and their unique characteristics.
Masters Degrees: Alumni Status Granted?
You may want to see also

In 1,1-Dibromoethene, the bromine atoms are on the same side of the double bond
Dibromoethene, also known as C2H2Br2, has three possible isomers. These isomers are different structural configurations of the same molecule, with the same chemical formula but differing bond structures and properties. One of these isomers is 1,1-Dibromoethene, where the two bromine atoms are on the same side of the carbon-carbon double bond.
In 1,1-Dibromoethene, the two bromine atoms are bonded to the same carbon atom. This carbon atom is, in turn, double-bonded to another carbon atom, which has two hydrogen atoms bonded to it. This configuration results in an unequal distribution of charge and a net dipole moment. The molecule has a dipole, meaning it has a partial positive or negative charge. This is because the bromine atoms, being more electronegative, attract the shared electrons in their bonds with carbon towards themselves, creating a partial negative charge. Conversely, the carbon atoms are left with a partial positive charge.
The presence of dipoles in a molecule can affect its physical and chemical properties, such as solubility and boiling point. In 1,1-Dibromoethene, the dipoles due to the C-Br bonds do not cancel each other out, resulting in a net dipole moment. This is in contrast to the other dibromoethene isomers, where the dipoles may balance each other out, resulting in no net dipole.
The isomers of dibromoethene, including 1,1-Dibromoethene, can be interconverted through chemical reactions. For example, the formation of 1,2-dibromoethane from ethene and bromine is an example of an addition reaction. In this reaction, the carbon-carbon double bond in ethene is broken, and a bromine atom is attached to each carbon atom by a single bond, resulting in a saturated compound. The reaction can be represented as:
${H}_{2}C=C{H}_{2}+Br_{2}\to {H}_{2}BrC-CBr{H}_{2}$
The isomers of dibromoethene, including 1,1-Dibromoethene, are important in understanding the behaviour of organic compounds and their reactions. The presence of dipoles and their resulting properties can influence the reactivity and applications of these molecules in various chemical processes.
UK Constitution: A Rulebook for the Nation?
You may want to see also
Explore related products

The C-Br bond in 1,1-dibromoethene is polar
Dibromoethene (C₂H₂Br₂) has three isomers: cis, trans, and 1,1-dibromoethene. The trans isomer has no net dipole moment, while the cis and 1,1 isomers do due to their different spatial arrangements of bromine atoms. The C-Br bond in 1,1-dibromoethene is polar due to the unequal distribution of charge and the resulting net dipole moment. This is because the two bromine atoms are attached to the same carbon atom, leading to a different spatial arrangement.
In general, the polarity of a bond is influenced by differences in electronegativity between the atoms involved. When one atom is more electronegative than another, it attracts electrons more strongly, resulting in an uneven distribution of electron density and a polar bond. While the cutoff for classifying a bond as non-polar is typically considered to be an electronegativity difference of less than 0.5, the C-Br bond only slightly exceeds this threshold, with carbon having a value of 2.55 and bromine having a value of 2.96 on the Pauling electronegativity scale. Nonetheless, the C-Br bond is still considered polar, and this polarity has been observed to affect electrophilic aromatic substitution reaction rates.
The polar nature of the C-Br bond in 1,1-dibromoethene can also be understood by examining the dipoles it creates. In this isomer, the dipoles due to the C-Br bonds do not cancel each other out, resulting in a net dipole moment. This is in contrast to the trans isomer of dibromoethene, where the symmetric arrangement of the bromine atoms cancels out the dipoles, resulting in a nonpolar molecule. The cis isomer, on the other hand, has a bent molecular structure, leading to a polar molecule.
The concept of molecular geometry further highlights the importance of polarity in chemical compounds. The arrangement of atoms within a molecule can significantly impact its overall polarity. In the case of dibromoethene isomers, the spatial arrangement of bromine atoms around the carbon-carbon double bond determines whether the molecule has a net dipole moment. This relationship between molecular geometry and polarity is not limited to dibromoethene but is a fundamental aspect of chemistry, influencing the properties and behaviours of a wide range of chemical compounds.
The Constitution's Safeguards for Judicial Independence
You may want to see also

The two bromine atoms in (Z)-1,2-Dibromoethene are on the same side of the double bond
There are three different possible structures, or isomers, of a dibromoethene molecule, C2H2Br2. The two bromine atoms might be on the same side of the double bond or on opposite sides of the double bond. This means that there are two structural isomers with this molecular formula: 1,1-dibromoethene, where the two bromine atoms are on the same side of the double bond, and 1,2-dibromoethene, where they are on opposite sides.
The (Z)-1,2-dibromoethene isomer is one of the three possible isomers of a dibromoethene molecule. In this isomer, the bromine atoms are on the same side of the double bond. This isomer contains a total of six atoms: two hydrogen atoms, two carbon atoms, and two bromine atoms. It also contains five bonds: three non-H bonds, one multiple bond, and one double bond. The molecular weight of (Z)-1,2-dibromoethene is calculated by multiplying the atomic weight of each constituent element by the number of atoms of that element in the molecular formula.
The molecular formula of (Z)-1,2-dibromoethene is C2H2Br2. The SMILES string of this isomer is BrC=CBr, which can be imported into most molecule editors for conversion into two-dimensional or three-dimensional models. The two-dimensional structure is also called a skeletal formula, which is the standard notation for organic molecules. The carbon atoms are implied to be located at the corners, and the hydrogen atoms attached to the carbon atoms are not indicated. Each carbon atom is considered to be associated with enough hydrogen atoms to provide the carbon atom with four bonds.
The (Z)-1,2-dibromoethene compound may be called different names depending on the various industrial applications. The molecule contains a total of five bonds: three non-H bonds, one multiple bond, and one double bond. The molecule has a net dipole moment because the dipoles from the C-Br bonds add together. This is in contrast to the (E)-1,2-Dibromoethene isomer, which has no net dipole moment because the dipoles from the C-Br bonds oppose each other.
National Anthem: Freedom to Sit or Stand?
You may want to see also