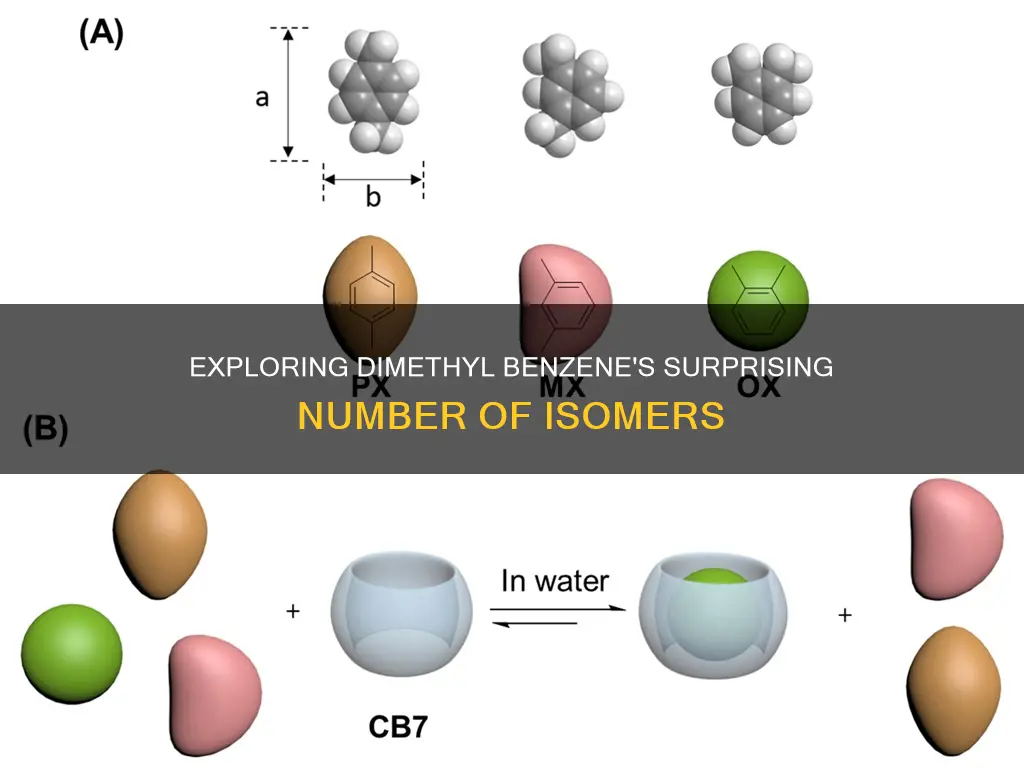
Dimethyl benzene, also known as xylene, has three constitutional isomers: ortho-xylene, meta-xylene, and para-xylene. These isomers differ in the number of unique carbon environments, with ortho-xylene having four, meta-xylene having five, and para-xylene having three. The presence of these unique carbon environments allows for the differentiation of the isomers through techniques such as carbon nuclear magnetic resonance (13C NMR) spectroscopy. While dimethyl benzene exhibits constitutional isomerism, it does not possess any configurational isomers due to the nature of its six-membered ring structure.
| Characteristics | Values |
|---|---|
| Number of constitutional isomers | 3 |
| Names of isomers | Ortho-xylene, meta-xylene, para-xylene |
| Number of unique carbon environments in ortho-xylene | 4 |
| Number of unique carbon environments in meta-xylene | 5 |
| Number of unique carbon environments in para-xylene | 3 |
Explore related products
What You'll Learn

Dimethyl benzene's three isomers
Dimethyl benzene, also known as xylene, has three isomers: ortho-xylene, meta-xylene, and para-xylene. These isomers differ in the number of unique carbon environments, which can be identified using a 13C NMR spectrum. Ortho-xylene has four unique carbon environments, meta-xylene has five, and para-xylene has three. This distinction is important because each unique carbon environment gives rise to a separate signal in the 13C NMR spectrum, allowing for the differentiation of the isomers based on the number of signals.
Ortho-xylene, or o-xylene, is an important precursor to phthalic anhydride. It is one of the isomers typically found in commercial or laboratory-grade xylene, which usually contains about 40-65% of m-xylene and up to 20% each of o-xylene, p-xylene, and ethylbenzene. Meta-xylene, or m-xylene, has a lower melting point compared to the other isomers, at -47.87 °C, and is less sought-after due to the relatively modest demand for isophthalic acid. Para-xylene, or p-xylene, has the highest melting point among the isomers at 13.26 °C and is the principal precursor to terephthalic acid and dimethyl terephthalate, which are monomers used in the production of PET plastic bottles and polyester clothing.
The different isomers of xylene have distinct properties and applications. The complexes formed by the different isomers can have significantly diverse characteristics. For example, p-xylene is highly valued and widely consumed in the production of plastics and polyester. The ratio of isomers can be manipulated to favour p-xylene through processes like the UOP-Isomar process or transalkylation of xylene with itself or trimethylbenzene. These processes are catalysed by zeolites, and ZSM-5 plays a role in facilitating isomerization reactions for mass-producing modern plastics.
Xylenes are formed through the methylation of toluene and benzene, and they exhibit specific reactions involving the methyl groups and the ring C–H bonds. The methyl groups, being benzylic, are susceptible to free-radical reactions, including halogenation to form xylene dichlorides or mono-bromination to produce xylyl bromide, a tear gas agent. Electrophilic attacks on the aromatic ring lead to the formation of chloro- and nitroxylenes. While xylene is flammable and can cause central nervous system depression upon inhalation, it has a modest acute toxicity, with LD50 values ranging from 200 to 5000 mg/kg in animals.
The Constitution's Anti-Tyranny Safeguards: Separation of Powers
You may want to see also

Ortho-xylene, meta-xylene, and para-xylene
Dimethyl benzene, also known as xylene, has three isomers: ortho-xylene, meta-xylene, and para-xylene. These structural isomers are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring. The position of the substituted hydrogens determines which isomer is formed.
The isomers also have distinct applications. Para-xylene is converted to terephthalic acid, which is used in the production of plastics. Ortho-xylene is a precursor to phthalate esters, which are also used as plasticizers. Meta-xylene, on the other hand, is converted to isophthalic acid derivatives, which are components of alkyd resins.
Xylene, as a mixture of these three isomers, has various industrial uses. It is a common solvent in printing, rubber, and leather industries, and it is used in the production of ink, rubber, adhesives, paints, and varnishes. Xylene is also used in dentistry to dissolve gutta-percha, a material used in root canal treatments. In the laboratory, xylene is employed as a solvent and as a cooling agent for reaction vessels.
White House Parties: Where Do They Happen?
You may want to see also

Differentiating isomers with 13C NMR
Dimethyl benzene has three isomers: ortho-xylene, meta-xylene, and para-xylene. Differentiating isomers using 13C NMR spectroscopy is a common practice in chemistry. This method involves examining the number of unique carbon environments in each isomer. Each unique carbon environment gives rise to a separate signal in the 13C NMR spectrum, allowing for the differentiation of isomers based on the number of signals. For example, ortho-xylene has four unique carbon environments, meta-xylene has five, and para-xylene has three.
The integration of the 13C NMR spectrum is also important for differentiation. For instance, a CH3 attached to an unsaturated functional group will show up around ppm= 1.8 with 3H, while a CH2 attached to Cl will appear around ppm= 4.0 with 2H. Alkenes typically absorb at 122 ppm and appear as sharp lines, making them easy to distinguish. The double-bonded carbons in alkene molecules also affect shifts in 13C NMR spectra.
Additionally, 13C NMR chemical shifts have been used to differentiate conformational isomers of metal-complexed and uncomplexed bispidine derivatives. In some cases, 1H-13C correlations can be ambiguous, and prior knowledge of 1H and/or 13C chemical shifts is necessary to avoid errors in isomer identification.
Furthermore, 13C{14N} solid-state NMR spectroscopy has been used to differentiate heterocyclic isomers, especially in highly substituted rings. The 14N-filtered 13C NMR spectra allow for clear differentiation between certain isomers, such as the 2- versus 4-pyridone core and the isoxazole and oxazole heterocyclic isomers.
While 13C NMR is a valuable tool for differentiating isomers, it is important to note that in some cases, such as with alkynel hydrogen atoms, proton NMR may be more effective. The coupling constant is smaller in a cis isomer, resulting in upfield signals, while trans isomers have larger coupling constants and yield downfield signals.
Who Protects the Treasury Secretary? Secret Service Involvement
You may want to see also
Explore related products

Dimethyl benzene vs Dewar benzene
Dimethyl benzene, also known as xylene, has three isomers: ortho-xylene, meta-xylene, and para-xylene. Xylene was first isolated and named in 1850 by French chemist Auguste Cahours, and it is produced mainly as part of the BTX aromatics (benzene, toluene, and xylenes) extracted from the product of catalytic reforming. It has a variety of industrial uses, including as a solvent in printing, rubber, and leather industries, and as a component of ink, rubber, and adhesives.
On the other hand, Dewar benzene, also known as bicyclo [2.2.0] hexa-2,5-diene, is a bicyclic isomer of benzene with the molecular formula C6H6. Unlike benzene, it is not flat because the carbons where the rings join are bonded to four atoms, leading to tetrahedral geometry. It was named after James Dewar, who included this structure in a list of possible C6H6 structures in 1869, although he supported the correct structure proposed by August Kekulé in 1865. Dewar benzene has been studied for its potential use as a protecting group for solid-state aryl-aryl interactions and in the synthesis of various compounds.
While both dimethyl benzene and Dewar benzene involve modifications to the benzene ring, they differ in their structures and applications. Dimethyl benzene has three isomers and is used in various industrial processes, while Dewar benzene is a bicyclic isomer of benzene that has been investigated for its potential in organic chemistry applications.
In terms of toxicity, xylene is flammable and has modest acute toxicity, with LD50 ranges from 200 to 5000 mg/kg for animals. The main effect of inhaling xylene vapor is depression of the central nervous system, with symptoms such as headache, dizziness, nausea, and vomiting. However, specific toxicity studies on Dewar benzene and its derivatives do not appear to be readily available from the sources provided.
Additionally, it is worth noting that Dewar benzene has considerable strain energy and reverts to benzene with a chemical half-life of two days. This thermal conversion is relatively slow due to symmetry forbidden by orbital symmetry arguments. This information highlights the stability and behavior of Dewar benzene over time.
The Living Constitution: Judicial Review's Dynamic Impact
You may want to see also

Benzene ring structures and isomers
Benzene, or C6H6, is an organic chemical compound composed of six carbon atoms joined in a planar hexagonal ring, with one hydrogen atom attached to each carbon atom. Due to its cyclic continuous pi bonds between carbon atoms, benzene is classed as an aromatic hydrocarbon. It is the first and simplest of the six-carbon cyclical hydrocarbons referred to as aromatic hydrocarbons.
The structure of benzene was first proposed by German chemist Friedrich August Kekulé in 1865. He suggested that the structure contained a ring of six carbon atoms with alternating single and double bonds. This symmetrical ring could explain why there appeared to be only one isomer of any monoderivative of benzene and exactly three isomers of every disubstituted derivative. These three isomers correspond to the ortho, meta, and para patterns of arene substitution.
The benzene molecule is easily identified using various techniques due to its unique symmetrical structure. For example, proton and carbon NMR spectroscopy of benzene produce only one signal (a singlet) because all the protons and carbons are chemically equal. This chemical equality also leads to the absence of the effects of coupling and splitting, which are typically seen in the NMR of other aromatic rings.
Dimethyl benzene, also known as xylene, is a derivative of benzene with two methyl groups attached to the benzene ring. There are three constitutional isomers of dimethyl benzene, commonly named ortho-xylene, meta-xylene, and para-xylene. These names correspond to the different positions of the two methyl groups on the benzene ring. Ortho-xylene has four unique carbon environments, meta-xylene has five, and para-xylene has three, which can be identified using 13C NMR spectroscopy.
Health, Negative Constitution, and You
You may want to see also
Frequently asked questions
Dimethyl benzene has three isomers: ortho-xylene, meta-xylene, and para-xylene.
Ortho-xylene is also known as 1,2-dimethylbenzene or 1,2-dideuteriobenzene.
Ortho-xylene has four, meta-xylene has five, and para-xylene has three.
Each unique carbon environment gives a separate signal in the 13C NMR spectrum, allowing differentiation between the isomers.
Polycyclic aromatic compounds (PACs) can have multiple isomers, and the number of theoretically possible isomers can be in the thousands.