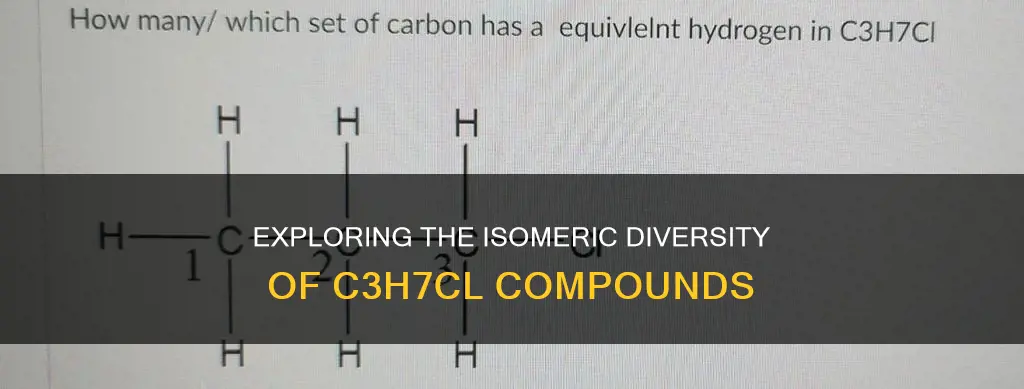
The compound C3H7Cl can have constitutional isomers. Constitutional isomers, also referred to as structural isomers, are compounds that share the same molecular formula but differ in the way their atoms are connected. C3H7Cl, a straight-chain alkyl chloride (propyl chloride), has three carbon atoms, seven hydrogen atoms, and a single chlorine atom. By rearranging the carbon atoms and considering different positions for the chlorine atom within the carbon chain, C3H7Cl can form two constitutional isomers: 1-chloropropane and 2-chloropropane. These isomers have distinct structures while maintaining the same molecular formula.
| Characteristics | Values |
|---|---|
| Number of possible constitutional isomers | 2 |
| Names of the isomers | 1-chloropropane and 2-chloropropane |
| Number of carbon atoms | 3 |
| Number of hydrogen atoms | 7 |
| Number of chlorine atoms | 1 |
Explore related products
$18.99 $19.99
What You'll Learn
- C3H7Cl has two isomers: 1-chloropropane and 2-chloropropane
- Constitutional isomers have the same molecular formula but different atom connectivity
- C3H7Cl is a straight-chain alkyl chloride (propyl chloride)
- It has three carbon atoms, seven hydrogen atoms, and one chlorine atom
- The isomers are formed by rearranging carbon atoms and changing their positions

C3H7Cl has two isomers: 1-chloropropane and 2-chloropropane
The compound C3H7Cl is also known as n-propyl chloride, which is a colourless, flammable chemical compound. It has a higher melting and boiling point than propane due to the presence of the heavy electronegative chlorine atom. 1-chloropropane, or n-propyl chloride, is the simplest asymmetric chloropropane, analogous to the symmetric 2-chloropropane.
1-chloropropane has been associated with acute toxic effects in various studies. For example, in a rat study, the lethal dose for 50% of the test subjects for oral ingestion was found to be greater than 2 grams per kilogram of body weight, indicating a relatively low acute toxicity when ingested by rats. Occupational exposure to 1-chloropropane may occur through inhalation and dermal contact in workplaces where the compound is produced or used. Additionally, the general population may be exposed to 1-chloropropane through inhalation of ambient air and dermal contact with vapors and products containing the compound.
Understanding isomerism is key to grasping the diversity of organic compounds. To identify all constitutional isomers for a given molecular formula, we need to consider different arrangements of atoms while maintaining the same molecular formula. In the case of C3H7Cl, the two isomers, 1-chloropropane and 2-chloropropane, have the same molecular formula but different structural arrangements.
How US Constitution Influences Global Business
You may want to see also

Constitutional isomers have the same molecular formula but different atom connectivity
Constitutional isomers, also referred to as structural isomers, are compounds that have the same molecular formula but different atom connectivity. They are isomers that can be differentiated by the way their atoms are connected, rather than by differences in their spatial arrangement.
To determine whether two molecules are constitutional isomers, you need to count the number of each atom in both molecules and examine how the atoms are arranged. For instance, the molecules with the molecular formula C6H12 can be connected in different ways to form cyclohexane or 1-hexene. There are also two ways to connect C6H12 to form 2-hexene and 3-methyl-1-pentene.
The compound C3H7Cl is a straight-chain alkyl chloride (propyl chloride) that can have constitutional isomers. Its molecule has three carbon atoms, seven hydrogen atoms, and one chlorine atom. By rearranging the carbon atoms in different ways or considering branching or different positions for the chlorine atom within the carbon chain, C3H7Cl can form constitutional isomers. One possible isomer of C3H7Cl is 1-chloropropane, and another is 2-chloropropane.
In contrast, some compounds cannot form constitutional isomers due to their fixed structure or the limited number of ways their atoms can be arranged. For example, CH3Cl, a simple molecule with one carbon atom and one chlorine atom, cannot have constitutional isomers as there is only one way to arrange these atoms. Similarly, C3H8, which is propane, cannot have constitutional isomers because changing the connectivity of the carbon atoms would result in the same molecule.
Muslim Congresswomen: Upholding the Constitution?
You may want to see also

C3H7Cl is a straight-chain alkyl chloride (propyl chloride)
C3H7Cl is a straight-chain alkyl chloride, also known as propyl chloride. It is a molecule that contains three carbon atoms, seven hydrogen atoms, and one chlorine atom. This molecule is unique in that it can form constitutional isomers, which are compounds with the same molecular formula but different arrangements of atoms. In the case of C3H7Cl, the carbon atoms can be rearranged in different ways, leading to the formation of isomers.
Constitutional isomers, also known as structural isomers, are an important concept in organic chemistry. They occur when the connectivity of atoms within a molecule changes, resulting in different structural arrangements while maintaining the same molecular formula. This phenomenon is observed in C3H7Cl due to the presence of multiple carbon atoms, which can be rearranged to form distinct isomers.
One example of a C3H7Cl isomer is 1-chloropropane, where the chlorine atom is attached to the first carbon atom in the chain. Another isomer is 2-chloropropane, where the chlorine atom is bonded to the second carbon atom. These isomers have the same molecular formula, C3H7Cl, but differ in the positioning of the chlorine atom, resulting in unique structural configurations.
The ability of C3H7Cl to form constitutional isomers highlights the versatility of this molecule. By manipulating the arrangement of atoms, specifically the carbon atoms, it is possible to create different structural isomers with distinct properties. This understanding of isomerism is crucial in fields such as chemistry and materials science, where the manipulation of molecular structures is essential for designing new compounds with specific characteristics.
In summary, C3H7Cl, a straight-chain alkyl chloride or propyl chloride, exhibits the fascinating property of isomerism. Its molecular structure allows for the rearrangement of carbon atoms, leading to the formation of constitutional isomers. This knowledge contributes to our understanding of molecular diversity and the creation of novel compounds with tailored attributes.
Capital Punishment for Treason: What Does the Constitution Say?
You may want to see also
Explore related products
$42.23 $49.99

It has three carbon atoms, seven hydrogen atoms, and one chlorine atom
The molecular formula C3H7Cl represents a compound with three carbon atoms, seven hydrogen atoms, and one chlorine atom. This compound is also known as propyl chloride and has the potential to form constitutional isomers. Constitutional isomers, also referred to as structural isomers, are compounds that share the same molecular formula but differ in the way their atoms are connected.
In the case of C3H7Cl, the carbon atoms can be rearranged in different ways, leading to the formation of isomers. Specifically, the chlorine atom can occupy different positions within the carbon chain, resulting in distinct isomeric structures. This flexibility in atomic arrangement sets C3H7Cl apart from other compounds that cannot form constitutional isomers due to their fixed connectivity.
There are two identified isomers of C3H7Cl: 1-chloropropane and 2-chloropropane. These isomers differ in the position of the chlorine atom along the propane chain. By varying the position of the chlorine atom, we can create different structural isomers while maintaining the same molecular formula.
To visualize these isomers, we can draw their structural formulas. For 1-chloropropane, one chlorine atom is attached to one of the three carbon atoms in the propane chain. In 2-chloropropane, the chlorine atom occupies a different carbon atom in the chain. These structural variations result in distinct isomers, showcasing the versatility of C3H7Cl in forming constitutional isomers.
In summary, the compound C3H7Cl, with three carbon atoms, seven hydrogen atoms, and one chlorine atom, exhibits the unique ability to form constitutional isomers. By rearranging the carbon atoms and altering the position of the chlorine atom, two identified isomers, 1-chloropropane and 2-chloropropane, are formed. This showcases the importance of understanding isomerism in organic chemistry and how subtle changes in atomic arrangement can lead to the creation of structurally distinct compounds.
The Four Powers: How the House Wields Control and Influence
You may want to see also

The isomers are formed by rearranging carbon atoms and changing their positions
In organic chemistry, isomers are molecules with the same molecular formula but different structural or spatial arrangements of atoms within the molecule. Isomers can be formed by rearranging carbon atoms and changing their positions, resulting in different connectivity of atoms while maintaining the same molecular formula. This process is known as structural or constitutional isomerism.
The compound C3H7Cl is an example of a molecule that can form constitutional isomers. It has three carbon atoms, seven hydrogen atoms, and one chlorine atom. By rearranging the carbon atoms and changing their positions, C3H7Cl can have two isomers: 1-chloropropane and 2-chloropropane. These isomers have the same molecular formula but differ in the position of the chlorine atom within the carbon chain.
The formation of isomers through rearranging carbon atoms and changing their positions is not limited to C3H7Cl. Another example is the molecule with the molecular formula C4H9OH, which can have two structural isomers. In one isomer, the hydroxyl group (-OH) is attached to the end of the carbon chain, while in the other isomer, it is attached to the middle of the chain. This change in the position of the hydroxyl group results in different connectivity of atoms, creating two distinct isomers with the same molecular formula.
Additionally, the concept of position isomerism further illustrates how isomers can be formed by rearranging carbon atoms and changing their positions. In position isomerism, the basic carbon skeleton remains the same, but functional groups are moved to different positions within the molecule. For instance, in the molecule C3H6O, the position of the hydroxyl group can vary, resulting in different functional groups and, consequently, different compounds, such as propanal (an aldehyde) or propanone (a ketone).
In summary, the formation of isomers by rearranging carbon atoms and changing their positions is a fundamental aspect of structural isomerism in organic chemistry. The compound C3H7Cl serves as a clear example of this phenomenon, with its ability to form constitutional isomers through the rearrangement of carbon atoms and the alteration of chlorine atom positions.
The Supreme Court: Constitution's Guardian
You may want to see also
Frequently asked questions
There are two constitutional isomers possible for C3H7Cl: 1-chloropropane and 2-chloropropane.
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivity of atoms.
To identify constitutional isomers, we need to examine the molecular formula and assess the potential for different structural arrangements while maintaining the same formula.