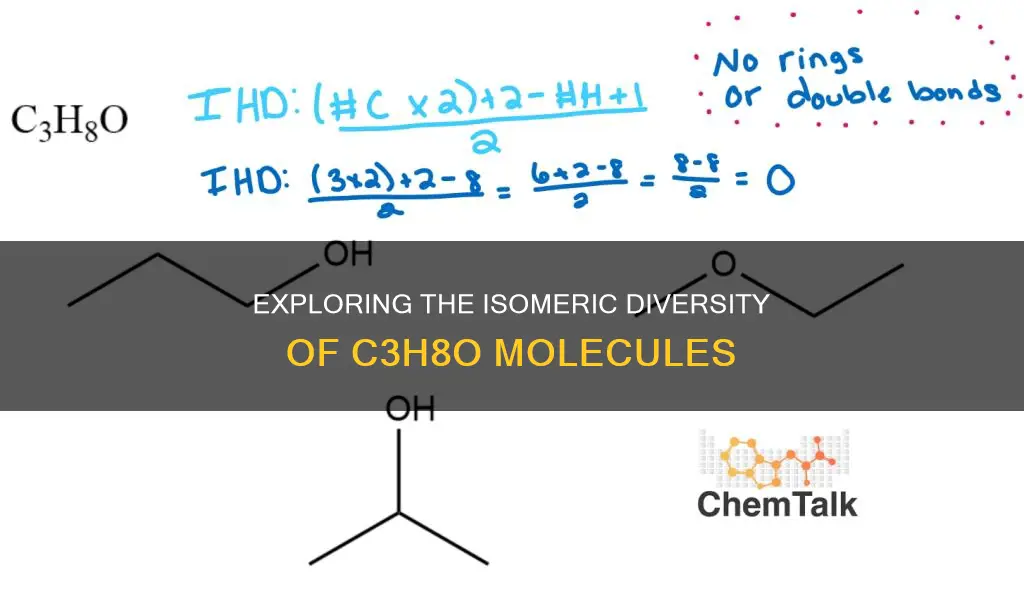
The molecular formula C3H8O has three constitutional isomers: 1-propanol, 2-propanol, and ethyl methyl ether. These isomers differ in the way their atoms are connected, with the same molecular formula. The isomers can be ranked in terms of their acidity, with ethyl methyl ether being the least acidic, followed by 2-propanol, and 1-propanol being the most acidic. This ranking is based on the stability of the conjugate bases formed upon proton donation.
| Characteristics | Values |
|---|---|
| Number of Constitutional Isomers | 3 |
| Names of Isomers | 1-propanol, 2-propanol, and ethyl methyl ether |
| Ranking in terms of Acidity | Ethyl methyl ether < 2-propanol < 1-propanol |
Explore related products
$18.99 $19.99
What You'll Learn

There are three constitutional isomers: 1-propanol, 2-propanol, and ethyl methyl ether
There are three constitutional isomers with the molecular formula C3H8O: 1-propanol, 2-propanol, and ethyl methyl ether (also known as methoxyethane).
Let's take a closer look at each of these isomers:
1-Propanol (or n-propanol) is a primary alcohol with the formula CH3CH2CH2OH. It is a colorless liquid with a characteristic alcoholic odor. 1-Propanol is soluble in water and commonly used as a solvent and intermediate in various chemical syntheses.
2-Propanol (or isopropyl alcohol) is a secondary alcohol with the formula CH3CHOHCH3. It is a colorless, flammable liquid with a strong odor. 2-Propanol is commonly used as a solvent and as a disinfecting agent due to its antimicrobial properties.
Ethyl methyl ether (or methoxyethane) is a colorless gaseous ether with the formula CH3OCH2CH3. Unlike other ethers such as dimethyl ether and diethyl ether, ethyl methyl ether has no current applications. However, its potential utility as an anesthetic and solvent has been investigated.
Constitutional isomers, or structural isomers, are compounds that have the same molecular formula but differ in the way their atoms are connected and arranged structurally. In this case, all three isomers have the formula C3H8O but differ in the arrangement of their carbon, hydrogen, and oxygen atoms, resulting in distinct chemical structures and properties.
Who's in the White House? Presidential Administration Explained
You may want to see also

Ethyl methyl ether is the least acidic of the three isomers
There are three constitutional isomers with the molecular formula C3H8O. These isomers are 1-propanol (n-propanol), 2-propanol (isopropanol), and ethyl methyl ether (methoxyethane).
The difference in acidity between these isomers can be attributed to their structural characteristics and the stability of their conjugate bases. Isomers, by definition, have the same molecular formula but differ in their structural formulas. This variation in structure leads to distinct physical and biological properties, such as differences in melting and boiling points.
For example, in the case of ethanol and dimethyl ether, both isomers have the formula C2H6O but differ in their physical states at room temperature. Ethanol is a liquid, while dimethyl ether is a gas due to its lower boiling point.
The acidity of ethers, such as ethyl methyl ether, can be affected by the presence of strong acids, like HI, which can protonate the ether oxygen. This protonation makes the oxygen a better leaving group, facilitating further reactions. However, the cleavage of ethers typically requires forcing conditions, such as strong acids and heat, or the use of alternative reagents like silanes at room temperature.
Deposit Checks: Apartment Contract Confusion?
You may want to see also

2-Propanol is a secondary alcohol
The compound 2-propanol, also known as isopropanol, isopropyl alcohol, or sec-propyl alcohol, is a secondary alcohol with the molecular formula C3H8O. It is a clear, colorless, flammable liquid with a mild odor. As a secondary alcohol, 2-propanol has an alcohol carbon atom attached to two other carbon atoms. This structure gives it unique properties and reactivity compared to other types of alcohols.
One of the key characteristics of 2-propanol is its miscibility with a wide range of substances. It is miscible with water, various organic solvents, and both polar and non-polar compounds. This versatility makes it a widely used solvent in chemical reactions, extractions, and chromatography techniques. For example, it is commonly employed in industrial applications such as antifreezes, quick-drying oils, and inks. Additionally, it serves as a solvent for gums, shellac, and essential oils.
In molecular biology, 2-propanol finds a specific application as a bioreagent for DNA and RNA precipitation from mammalian tissues. It is also used as a mobile phase in ion-exchange chromatography and high-performance liquid chromatography (HPLC). Furthermore, 2-propanol can be utilized as a drying agent or desiccant and as a carrier for delivering catalysts or reagents in synthetic reactions.
The synthesis of 2-propanol can be achieved through the hydration of propene or the hydrogenation of acetone. It was first synthesized in 1853 by Alexander William Williamson. Today, it is produced on a large scale, with over a million tonnes being manufactured annually worldwide.
In terms of safety, while death from ingestion or absorption of 2-propanol is rare, it is important to handle it with caution. Isopropyl alcohol is more toxic than ethanol and can act as a central nervous system depressant. Poisoning symptoms include flushing, headache, dizziness, nausea, vomiting, anesthesia, hypothermia, low blood pressure, respiratory depression, and coma. Additionally, isopropyl alcohol vapor is flammable and should be kept away from heat, sparks, and open flames.
Citing the Constitution: In-Text Style Guide
You may want to see also
Explore related products
$42.23 $49.99

1-Propanol is the most acidic of the three isomers
Propanol is an alcohol compound with the molecular formula C3H8O. It has two isomers: 1-propanol and 2-propanol. These isomers are examples of structural isomerism, where molecules have the same molecular formula but different structures or arrangements of atoms. In the context of propanol, the molecular formula indicates that any isomer must consist of exactly three carbon atoms, eight hydrogen atoms, and one oxygen atom.
The propanol molecule can occur in two different forms, depending on the position of the hydroxyl group (OH) on the carbon backbone. 1-Propanol has its hydroxyl group attached to the terminal or first carbon atom of the molecule, making it a primary alcohol. As a primary alcohol, it forms aldehydes when it undergoes oxidation and is more acidic than other alcohol categories. 2-Propanol, on the other hand, has its hydroxyl group attached to the middle or second carbon atom, classifying it as a secondary alcohol. Secondary alcohols are generally more reactive, more stable, and less acidic than 1-propanol.
The seemingly minor change in the position of the hydroxyl group leads to significant differences in the physical and chemical properties of 1-propanol and 2-propanol. This change in position influences the molecule's solubility and boiling point. The different connectivity in their structures results in distinct chemical behaviours and uses for these molecules. For example, 1-propanol is commonly used as a solvent in the pharmaceutical industry, while 2-propanol, also known as isopropyl alcohol (IPA) or isopropanol, has a wide range of applications across many industries.
In conclusion, 1-propanol is the most acidic of the two isomers of propanol due to its classification as a primary alcohol and the position of its hydroxyl group on the terminal carbon atom. This structural difference leads to variations in chemical behaviour and applications, highlighting the importance of understanding isomerism in organic chemistry.
Constitutional Isomers of Methocarbamol: Exploring Chemical Alternatives
You may want to see also

The isomers can be ranked in terms of acidity
The molecular formula C3H8O has three constitutional isomers: 1-propanol, 2-propanol, and ethyl methyl ether. These isomers differ in the way their atoms are connected. The ranking of these isomers in terms of acidity depends on the stability of the conjugate bases formed upon proton donation.
Ethyl methyl ether is the least acidic of the three isomers. This is because it only has C-H bonds. However, it should still be more acidic than an alkane (pKₐ ≈ 50) due to the electron-withdrawing effect of the oxygen atom. The expected acidity of ethyl methyl ether is pKₐ ≈ 30.
The two alcohol isomers, 1-propanol and 2-propanol, are more acidic than ethyl methyl ether due to the presence of the O-H bond, which has a highly electron-withdrawing effect. Typically, alcohols have a pKₐ of approximately 17.
1-Propanol, or n-propanol, is a linear alcohol with the structure CH3−CH2−CH2−OH. It has a hydroxyl group (-OH) attached to the first carbon. 2-Propanol, also known as isopropanol, has the structure CH3−CHOH−CH3, with the hydroxyl group attached to the second carbon, making it a secondary alcohol. The alkyl groups in 1-propanol act as electron donors, increasing the electron density in the OH bond and making the acid weaker.
Based on their structures, the isomers can be ranked in terms of increasing acidity as follows: ethyl methyl ether < 2-propanol < 1-propanol. This ranking is consistent with the stability of their conjugate bases.
Alexander Hamilton's Influence on the US Constitution
You may want to see also











































