Isomerism is a process that involves the presence of isomers, which are compounds with the same molecular formula but different structural formulas and arrangements. In the case of $C_{4}H_{8}Cl_{2}$, there may be multiple isomers due to the presence of four carbon atoms, which typically have a valency of four and can form four bonds with other atoms. Additionally, chlorine atoms have a valency of one and form one bond with other atoms. These factors contribute to the potential for various structural arrangements and isomers of $C_{4}H_{8}Cl_{2}$.
| Characteristics | Values |
|---|---|
| Chemical Formula | C4H8Cl2 |
| Common Name | 1,2-dichlorobutane |
| Number of Constitutional Isomers | 2 |
| Functional Group | Alkane |
| Halogen Substituent | Chlorine |
| Molecular Weight | 133.01 g/mol |
| Boiling Point | 161-163 °C |
| Melting Point | -45 °C |
| Density | 1.1 g/mL |
| Solubility | Soluble in organic solvents |
| Flash Point | 52 °C |
Explore related products
$18.99 $19.99
What You'll Learn
- Isomers have the same molecular formula but different structural formulas
- Stereoisomerism: compounds with the same molecular formula but different spatial orientation
- Structural isomerism: functional, chain, and tautomer isomers
- Isomerism classification: structural, functional, stereo, and geometrical isomers
- Identifying isomers: write down the IUPAC name of the compound

Isomers have the same molecular formula but different structural formulas
Isomers are compounds with the same molecular formula but different structural formulas and arrangements in space. This phenomenon is known as isomerism. For example, 1-propanol and 2-propanol are positional isomers as they differ in the position of double bonds or functional groups on the "parent" molecule (propane, in this case).
There are two main forms of isomerism: structural (or constitutional) isomerism and stereoisomerism (or spatial isomerism). In structural isomerism, the bonds between atoms differ, resulting in distinct arrangements of atoms in space. In stereoisomerism, the bonds are the same, but the relative positions of the atoms differ. Stereoisomers can be further classified into enantiomers and diastereomers. Enantiomers are non-superimposable mirror images, like a pair of hands, while diastereomers include all other forms of stereoisomers.
Conformational isomers, also known as conformers, differ in their rotation around a single bond. This occurs freely around single carbon-carbon bonds but not with double or triple bonds, which are "locked" in their orientation. Geometric isomerism is a type of isomerism where the order of atom bonding is the same, but the arrangement of atoms in space is different. For example, 2-butene has two geometric isomers: cis-2-butene and trans-2-butene. The cis isomer has two single hydrogen atoms on the same side, while the trans isomer has them on opposite sides.
The compound $C_{4}H_{8}Cl_{2}$ has possibly 13 isomers. However, it is not clear from the sources if these are all constitutional isomers or a combination of constitutional and stereoisomers.
Voting Power to Change the Constitution: How Many States?
You may want to see also

Stereoisomerism: compounds with the same molecular formula but different spatial orientation
Stereoisomerism is a form of isomerism in which molecules have the same molecular formula and sequence of bonded atoms, but differ in their three-dimensional orientations in space. This means that stereoisomers have the same chemical composition, but their structures differ in spatial arrangement.
Stereoisomers are often classified as either enantiomers or diastereomers. Enantiomers are stereoisomers that are related to each other by a reflection, like mirror images that are non-superposable. They have the same physical properties, except for the direction in which they rotate polarized light and how they interact with different enantiomers of other compounds. For example, human hands are a macroscopic analogy for this, as they are the same but opposite. Chiral local anaesthetics, such as ropivacaine and levobupivacaine, are enantiomers that exhibit reduced systemic toxicity compared to racemic mixtures.
Diastereomers, on the other hand, are stereoisomers that are not related as mirror images. They seldom have the same physical properties. An example is the meso form of tartaric acid, which forms a diastereomeric pair with levo- and dextro-tartaric acids. These, in turn, form an enantiomeric pair.
Stereoisomers can also be classified as geometric isomers or optical isomers. Geometric isomers include cis–trans isomers, E-Z isomers, and atropisomers, which arise due to restricted rotation about a double bond. Optical isomers, also known as enantiomers, are asymmetric molecules that rotate the plane of polarized light differently depending on the sequence of atoms around the chiral centre. They are designated as D/L, R/S, or +/− isomers.
The number of possible stereoisomers for a compound can be determined using the Le Bel-van't Hoff rule, which states that for a structure with n asymmetric carbon atoms, there is a maximum of 2n different stereoisomers. For example, D-glucose, with the formula C6H12O6, is expected to have a maximum of 4 stereoisomers.
Equality in the Constitution: A Search for the Word
You may want to see also

Structural isomerism: functional, chain, and tautomer isomers
Structural isomerism refers to molecules that share the same molecular formula but differ in the arrangement of atoms. This can manifest in several ways, including functional isomerism, chain isomerism, and tautomerism.
Functional isomers, also known as positional isomers, occur when the basic carbon skeleton remains the same, but functional groups are rearranged. For instance, consider the molecular formula C3H7Br. There are two possible isomers: one where the bromine atom sits at the end of the chain and the other where it's attached in the middle. Another example is C4H9OH, where the hydroxyl group can occupy different positions on the carbon chain.
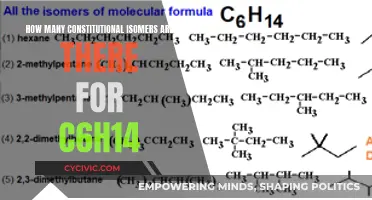
Chain isomerism, on the other hand, involves differences in how carbon atoms are bonded together, leading to branched or unbranched structures. This type of isomerism is observed in alkane molecules with four or more carbon atoms. For example, butane (C4H10) can exist as a straight-chain isomer or a branched isomer. Pentane (C5H12) also exhibits chain isomerism, with one unbranched "straight chain" isomer and two branched isomers.
Tautomers are a specific type of structural isomer that can readily interconvert through a process called tautomerization, often involving the relocation of a hydrogen atom within the compound. This relocation can occur through different mechanisms, such as prototropic tautomerism, ring-chain tautomerism, and valence tautomerism. Prototropic tautomerism, the most common form, can be considered a subset of acid-base behavior, where tautomers exhibit different protonation states with the same empirical formula and total charge. Ring-chain tautomerism involves the movement of a proton accompanied by a structural shift from an open structure to a ring, as seen in sugars like pyranose and furanose. Valence tautomerism, distinct from prototropic tautomerism, involves the rapid formation and rupture of single and/or double bonds without atom migration.
The Electoral College: Original Constitution or Later Addition?
You may want to see also
Explore related products

Isomerism classification: structural, functional, stereo, and geometrical isomers
Isomers are molecules that have the same molecular formula but differ in the arrangement of atoms in space. There are four main types of isomerism: structural isomerism, functional isomerism, stereoisomerism, and geometrical isomerism.
Structural isomerism
Structural isomerism, also known as constitutional isomerism, occurs when the atoms in the molecules of isomers are linked in different ways. In other words, the order of atom bonding differs. For example, butane (C4H10) has two structural isomers: one with a straight chain of carbon atoms and another with a branched chain. Another example is C3H7Br, which has two structural isomers: one with the bromine atom at the end of the chain and the other with the bromine atom attached in the middle.
Functional isomerism
Functional isomerism, also known as functional group isomerism, refers to compounds that share the same chemical formula but have different functional groups attached to them. For instance, C4H10O can be represented as ethoxyethane (C2H5OC2H5) or methoxy-propane (CH3OC3H7). Functional isomers are a type of structural isomer, as they have the same molecular formula but differ in the way atoms are connected.
Stereoisomerism
Stereoisomerism, also known as spatial isomerism, refers to molecules that have the same molecular formula and sequence of bonded atoms, but differ in the three-dimensional orientations of their atoms in space. Stereoisomers are further classified into enantiomers and diastereomers. Enantiomers are stereoisomers that are related to each other by a reflection, meaning they are mirror images of each other that are not superimposable. Diastereomers, on the other hand, are stereoisomers that are not related by a reflection and are not mirror images of each other. They occur when stereoisomers have different configurations at one or more equivalent stereocenters.
Geometrical isomerism
Geometrical isomerism, also known as cis-trans isomerism, occurs in compounds with restricted rotation, typically due to the presence of a carbon-carbon double bond. In geometrical isomers, the order of atom bonding is the same, but the arrangement of atoms in space is different. For example, 1,2-dichloroethene has two geometrical isomers: cis-1,2-dichloroethene and trans-1,2-dichloroethene.
A US Presidential Term: Duration and Limits
You may want to see also

Identifying isomers: write down the IUPAC name of the compound
To identify the isomers of a compound, we must first establish its IUPAC name. In this case, we are dealing with the compound $C_{4}H_{8}Cl_{2}$.
The compound $C_{4}H_{8}Cl_{2}$ has a four-carbon chain as its base structure, which can be butane or its branched isomers. This is an important first step in identifying the isomers of the compound.
Now, let's delve into the different types of isomers that can be derived from this compound. There are two main categories: structural isomers and stereoisomers.
Structural Isomers
Structural isomers are compounds that share the same molecular formula but differ in their structural formulas. In the context of $C_{4}H_{8}Cl_{2}$, we can identify two types of structural isomers: straight-chain isomers and branched isomers.
Straight-chain Isomers:
- 1,1-Dichlorobutane: CH3-CH2-C(Cl)(Cl)-CH3
- 1,2-Dichlorobutane: CH3-CH(Cl)-CH(Cl)-CH3
- 1,3-Dichlorobutane: CH3-CH(Cl)-CH2-CH(Cl)-CH3
- 1,4-Dichlorobutane: CH2(Cl)-CH2-CH2-CH(Cl)-CH3
- 2,2-Dichlorobutane: CH3-C(Cl)(Cl)-CH2-CH3
- 2,3-Dichlorobutane: CH3-CH(Cl)-C(Cl)-CH3
Branched Isomers:
- 1,1-Dichloro-2-methylpropane: CH3-C(Cl)(Cl)-CH(CH3)-CH3
- 1,2-Dichloro-2-methylpropane: CH3-C(Cl)-C(Cl)-CH3
- 1,3-Dichloro-2-methylpropane: CH3-CH(Cl)-C(Cl)-CH3
Stereoisomers
Stereoisomers have the same molecular formula and structural formula but differ in their spatial arrangement. Under stereoisomers, we find geometry and optical isomers.
In summary, by understanding the carbon skeleton and the placement of chlorine atoms, we can identify the various isomers of $C_{4}H_{8}Cl_{2}$. The compound exhibits a range of structural and stereoisomers, each with unique arrangements and spatial orientations.
Trump's Pardon of Arpaio: A Constitutional Violation?
You may want to see also
Frequently asked questions
Isomers are compounds with the same molecular formula but different structural formulas and arrangements.
There are three constitutional isomers in C4H8Cl2.
To identify the isomers of a compound, you should write down the IUPAC name of the compound.