Dibromobenzene is an organic compound with three constitutional isomers: 1,2-dibromobenzene (or o-dibromobenzene), 1,3-dibromobenzene (or m-dibromobenzene), and 1,4-dibromobenzene (or p-dibromobenzene). These isomers differ in the positioning of the two bromine atoms on the benzene ring. The synthesis of these isomers and their reactions, such as chlorination and nitration, are important topics in organic chemistry, and they have various applications in chemical synthesis and the production of derivatives.
| Characteristics | Values |
|---|---|
| Number of Constitutional Isomers | 3 |
| Names of Isomers | 1,2-Dibromobenzene (o-Dibromobenzene), 1,3-Dibromobenzene (m-Dibromobenzene), 1,4-Dibromobenzene (p-Dibromobenzene) |
| Molecular Formula | C6H4Br2 |
| Molecular Weight | 233.93 g/mol |
| Melting Point | Isomer-specific: o-Dibromobenzene - 44.5 °C, m-Dibromobenzene - 53-55 °C, p-Dibromobenzene - 103-105 °C |
| Boiling Point | Isomer-specific: o-Dibromobenzene - 280 °C, m-Dibromobenzene - 279 °C, p-Dibromobenzene - 284 °C |
| Density | Isomer-specific: o-Dibromobenzene - 1.9 g/cm³, m-Dibromobenzene - 1.8 g/cm³, p-Dibromobenzene - 1.7 g/cm³ |
| Solubility | Isomer-specific, but generally low solubility in water, higher in organic solvents |
| Symmetry | Isomer-specific: o-Dibromobenzene has a plane of symmetry, m-Dibromobenzene has a symmetric carbon, p-Dibromobenzene has two planes of symmetry |
| NMR Spectroscopy | Isomer-specific, but characteristic peaks for bromine substituents |
| IR Spectroscopy | Absorption bands characteristic of aromatic C-Br stretches |
| UV-Vis Spectroscopy | Isomer-specific, but generally absorb in the UV range due to π-π* transitions |
| Fluorescence | Weak fluorescence in the blue region, isomer-specific variations |
Explore related products
What You'll Learn

Dibromobenzene has three isomers
In the case of dibromobenzene, the three isomers arise from the different positions of the two bromine atoms on the benzene ring. The benzene ring is a hexagonal structure made up of six carbon atoms, and each carbon atom can form up to four bonds with other atoms. This property of carbon, known as catenation, allows it to form chains and cyclic structures, such as the benzene ring.
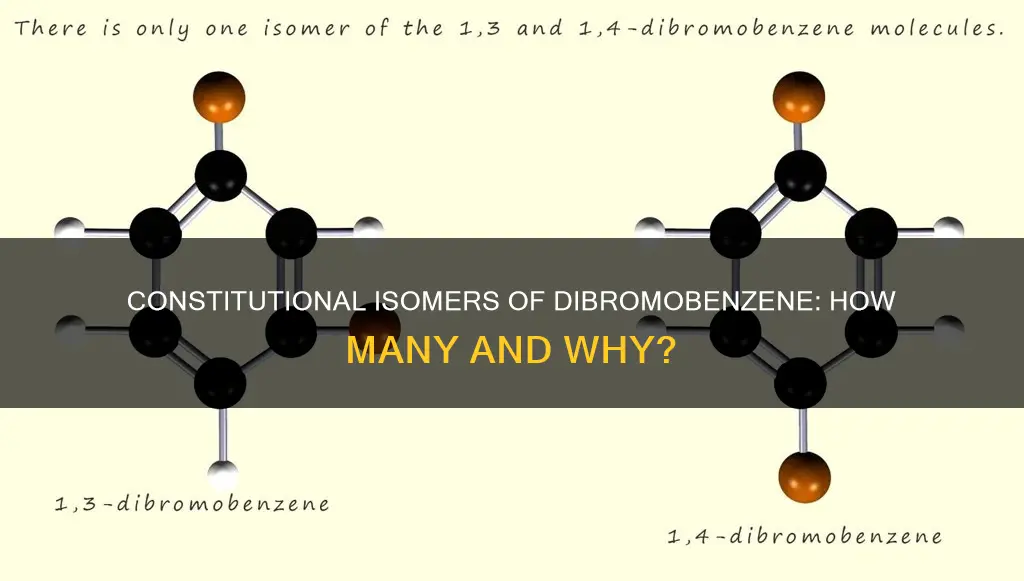
The three isomers of dibromobenzene differ in the arrangement of the bromine atoms relative to each other on the benzene ring. In 1,2-dibromobenzene, the two bromine atoms are located on adjacent carbon atoms, resulting in an "ortho" arrangement. In 1,3-dibromobenzene, the bromine atoms are positioned on carbon atoms that are separated by one carbon atom, forming a "meta" configuration. Finally, in 1,4-dibromobenzene, the bromine atoms occupy positions on carbon atoms that are two carbon atoms apart, resulting in a "para" orientation.
These isomers of dibromobenzene can be formed through various chemical reactions, such as chlorination, nitration, and fermentation processes. For example, the whole-cell fermentation of m-dibromobenzene yields a unique compound with two chemically distinct vinylic bromine atoms. Additionally, the synthesis of m-dibromobenzene involves introducing bromine directly onto the benzene ring through a reaction with bromine and FeBr3. Understanding the directing characteristics of substituents, such as nitro and chloro groups, is crucial in controlling the formation of specific isomers during synthetic processes.
Bribery: Criminal Liability in Offering and Accepting Bribes
You may want to see also

Aromatic isomers
Dibromobenzene is an organic compound with the formula C6H4Br2. It is a derivative of benzene with two bromine atoms substituted for hydrogen atoms. The arrangement of these bromine atoms on the benzene ring gives rise to the concept of aromatic isomers.
In chemistry, isomers are compounds that have the same molecular formula but differ in the arrangement of their atoms, resulting in distinct structural formulas. Dibromobenzene, with its two bromine atoms, can have these atoms arranged in different positions on the benzene ring, leading to the formation of isomers.
The isomers of dibromobenzene are classified as ortho, meta, or para, depending on the relative positions of the bromine atoms on the ring. In the ortho isomer, the bromine atoms are adjacent to each other, occupying the 1 and 2 positions on the ring. In the meta isomer, the bromine atoms are separated by one carbon atom, occupying the 1 and 3 positions. Finally, in the para isomer, the bromine atoms are directly across from each other, occupying the 1 and 4 positions.
There are three aromatic isomers of dibromobenzene: ortho, meta, and para. The meta isomer (m-dibromobenzene) is considered unique and challenging to synthesise because introducing a bromine group onto the benzene ring typically results in a mixture of ortho and para isomers, with the meta isomer forming in very small amounts. However, through specific procedures, such as converting a nitro group to a bromo group and using certain reagents, the synthesis of m-dibromobenzene can be achieved.
Congress' Power to Levy Taxes: Constitutional Basis?
You may want to see also

o-dibromobenzene
Dibromobenzene has three isomers, including o-dibromobenzene (or 1,2-dibromobenzene), 1,3-dibromobenzene, and 1,4-dibromobenzene. This response will focus on o-dibromobenzene, one of the isomers of dibromobenzene.
Structure and Properties of o-Dibromobenzene
Synthesis of o-Dibromobenzene
The synthesis of o-dibromobenzene typically involves bromination of benzene. This reaction occurs when benzene undergoes electrophilic aromatic substitution with liquid bromine (Br2) in the presence of a Lewis acid catalyst, such as iron or aluminium bromide. During this process, the bromine molecule acts as the electrophile, and the catalyst serves to polarize the bromine molecule, facilitating its attack on the benzene ring.
Applications and Uses
Safety Considerations
Like many brominated compounds, o-dibromobenzene should be handled with care. It is classified as a hazardous substance and may cause skin and eye irritation, as well as potential respiratory issues if inhaled. Proper protective equipment, adequate ventilation, and adherence to safety protocols are crucial when working with this compound.
Electoral Timetables: Constitutional Requirements for Elections
You may want to see also
Explore related products

m-dibromobenzene
Dibromobenzene has three isomers, one of which is m-dibromobenzene, or 1,3-dibromobenzene. This isomer is a colourless liquid at room temperature and is an aryl bromide. It can be prepared by diazotization of 3-bromonitrobenzene, followed by a Sandmeyer reaction with cuprous bromide.
The compound is tetravalent, meaning it can form many compounds with various properties. Carbon has the unique property of catenation, allowing carbon atoms to form large, stable chains and cyclic structures that exhibit a wide range of properties. One example of this is benzene, a six-membered ring compound that exhibits aromaticity.
The electrons in the benzene atom are not stationary but are continuously resonating. This resonance effect is one of several operating in organic compounds. Another is the inductive effect, which is caused by the transmission of inequality in electron sharing during the bonding process through the chain of atoms in the molecule. This effect can result in a permanent dipole in the compound.
The Constitution: Colonies' Freedom from Great Britain
You may want to see also

p-dibromobenzene
Dibromobenzene, or C6H4Br2, has three isomers. One of these is 1,4-Dibromobenzene, also known as p-dibromobenzene, which is an aryl bromide and isomer of dibromobenzene that is solid at room temperature. It has a strong smell, similar to that of the lighter chlorine analogue.
The compound also finds use in the development of advanced materials. For instance, it can be utilized in the synthesis of polymers or composite materials. The presence of bromine atoms in p-dibromobenzene can impart unique properties to the resulting materials, such as flame retardancy or specific optical characteristics.
Additionally, p-dibromobenzene can be employed in chemical research and the development of new reactions. It can act as a model compound for studying the reactivity of brominated aromatic compounds. The symmetrical structure of p-dibromobenzene makes it a useful substrate for investigating the selective functionalization of aromatic rings.
In terms of safety considerations, p-dibromobenzene is generally considered a hazardous substance. It is important to handle it with appropriate precautions to minimize potential risks. Proper ventilation or respiratory protection may be required during its use to avoid inhalation of vapors. Skin and eye irritation may occur, so protective gloves and eyewear are recommended. Spills or releases of p-dibromobenzene should be contained and disposed of properly to prevent environmental contamination.
The Preamble's Promise: Insuring Domestic Tranquility's Importance
You may want to see also
Frequently asked questions
There are three constitutional isomers for dibromobenzene: 1,2-dibromobenzene (o-dibromobenzene), 1,3-dibromobenzene (m-dibromobenzene), and 1,4-dibromobenzene (p-dibromobenzene).
The differences between these isomers lie in the positioning of the bromine atoms on the benzene ring. 1,2-dibromobenzene has bromine atoms at the 1 and 2 positions, 1,3-dibromobenzene has them at the 1 and 3 positions, and 1,4-dibromobenzene has them at the 1 and 4 positions.
Yes, each isomer has unique reactions and properties. For example, 1,2-dibromobenzene is a precursor to many 1,2-disubstituted derivatives of benzene, such as 1,2-dicyanobenzene and dithioethers. Meanwhile, the synthesis of m-dibromobenzene involves a unique procedure, and it has been used in the preparation of certain natural products through reactions like the Diels-Alder cycloaddition.
Pure 1,2-dibromobenzene is a colorless liquid, while impure samples may appear yellowish. I couldn't find specific information on the physical appearances of the other two isomers.
Yes, there are also stereoisomers of dibromobenzene. These stereoisomers differ in the arrangement of atoms or groups in three-dimensional space, but they have the same constitutional structure.