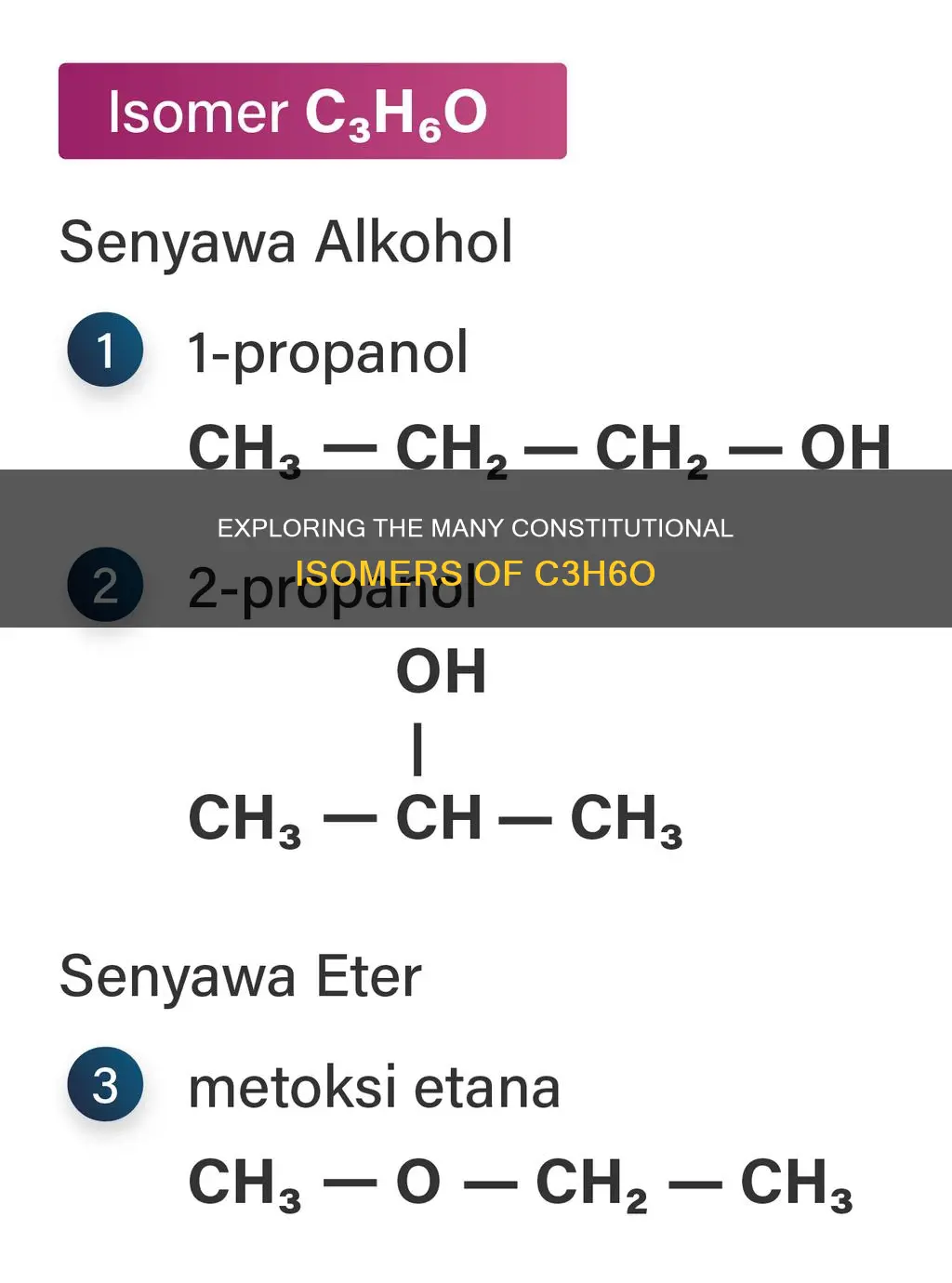
The molecular formula C3H6O represents a class of organic compounds known as hemiterpenes, which are a type of terpene with one isoprene unit. These compounds are of interest in fields such as perfumery and flavour chemistry due to their characteristic strong odours. When considering the number of constitutional isomers of C3H6O, it is important to note that the arrangement of atoms in these isomers must be different, but the molecular formula remains the same. There are two possible constitutional isomers for C3H6O.
| Characteristics | Values |
|---|---|
| Number of constitutional isomers | 2 |
Explore related products
What You'll Learn

There are two possible structural isomers of C3H6O
The molecular formula C3H6O represents a compound with three carbon atoms, six hydrogen atoms, and one oxygen atom. This compound has two possible structural isomers. Structural isomers are molecules with the same molecular formula but different bonding patterns and atomic arrangements.
To determine the number of structural isomers, we must first calculate the Double Bond Equivalent (DBE). The formula for DBE is:
$$ \text {DBE} = \frac{(2 \times \text{number of carbon atoms} + 2 - \text{number of hydrogen atoms})}{2} $$
Plugging in the values for C3H6O, we get:
$$ \text{DBE} = \frac{(2 \times 3 + 2 - 6)}{2} = \frac{2}{2} = 1 $$
This indicates the presence of either one double bond or one ring in the structure. With this information, we can identify the possible functional groups: alcohols (–OH), ethers (R–O–R'), aldehydes (R–CHO), and ketones (R–C(=O)–R).
Considering the DBE and the possible functional groups, we can construct the two structural isomers:
Isomer 1: Alkenes with the –OH group
One possible isomer is an alkene with the –OH group, specifically prop-1-en-1-ol. In this isomer, the double bond is between two carbon atoms, and the oxygen atom is part of the –OH group.
Isomer 2: Compound with a ring structure
The other isomer could have a ring structure, such as oxacyclobutane or cyclopropanol. In this isomer, the oxygen atom is part of the ring, forming a stable structure.
In summary, by analyzing the molecular formula, calculating the DBE, identifying functional groups, and constructing possible structures, we conclude that there are two structural isomers of C3H6O. These isomers differ in their bonding patterns and atomic arrangements, showcasing the versatility of chemical compounds with the same molecular formula.
The Constitution and Women: A Mention or Omission?
You may want to see also

These isomers should be drawn out to show their electron dot structures
The molecular formula C3H6O represents compounds with three carbon atoms, six hydrogen atoms, and one oxygen atom. There are two possible isomers for this molecular formula: propanal and propanone (also known as acetone).
Propanal is an aldehyde with the structural formula CH3CH2CHO. It consists of a three-carbon chain with a terminal aldehyde functional group (─CHO). The electron-dot structure for propanal would show the arrangement of carbon, hydrogen, and oxygen atoms, with a double bond between one carbon and oxygen atom, and single bonds connecting the other carbon and hydrogen atoms.
C • H - C • H2 - C • H = O •
Propanone, on the other hand, is a ketone with the structural formula CH3COCH3. It features a three-carbon chain with a carbonyl group (C=O) in the middle. Its electron-dot structure would depict the arrangement of carbon, hydrogen, and oxygen atoms, highlighting the double bond between one carbon and oxygen atom, as well as the single bonds connecting the other carbon and hydrogen atoms.
The electron-dot structure for propanone is as follows:
C • H3 - C • H = O = C • H3
These isomers, propanal and propanone, demonstrate the structural diversity achievable with the same molecular formula, showcasing the versatility of organic compounds. The electron-dot structures illustrate the distinct bonding patterns and connectivity of atoms in each isomer, highlighting their unique chemical properties and functionalities.
The Constitution: Freedom or Control?
You may want to see also

The degrees of unsaturation must be determined
To determine the degrees of unsaturation, it is important to understand what is meant by "unsaturation" and "saturation". A saturated molecule contains only single bonds and no rings. In other words, it has the maximum number of hydrogen atoms possible for an acyclic alkane. The formula for this is 2C+2, where C represents the number of carbons. For example, for the molecular formula C3H4, the number of actual hydrogens needed for the compound to be saturated is 8 (2C+2=(2x3)+2=8).
The degree of unsaturation, also known as the Hydrogen Deficiency Index, is a tool used by chemists to identify unknown compounds. It is particularly useful when the molecular formula does not provide enough information about the structure of the compound. The degree of unsaturation is the total sum of pi bonds and ring structures in the molecule. It is calculated using the formula:
> Unsat = C + 1 - \frac{H + X - N}{2}
Where C is the number of carbons, H is the number of hydrogens, X is the number of halogens, and N is the number of nitrogens. When calculating the degrees of unsaturation, the number of halogens and nitrogens must be taken into account. Halogens, such as chlorine or bromine, replace hydrogen, so for each halogen present, one hydrogen is added to the total. Nitrogen, on the other hand, provides two additional spaces for hydrogen, resulting in a net gain of one spot for hydrogen bonding. Thus, for a structure to be fully saturated, it must have one additional hydrogen per nitrogen.
Additionally, the presence of oxygen and sulfur does not affect the degree of unsaturation, so these elements are ignored in the formula. For example, in the formula C8H6F3NO2, the oxygens are ignored, and the fluorine (a halogen) and nitrogen are taken into account, resulting in a reduced equation of C8H6+3–1 = C8H8. This formula has five degrees of unsaturation.
In summary, determining the degrees of unsaturation involves substituting all non-hydrogen and non-carbon atoms in the molecular formula, then applying the formula to calculate the degree of unsaturation. This provides valuable information about the structure of an unknown compound.
George Washington: Constitution's Lifeblood
You may want to see also

The formula for degrees of unsaturation: Unsat = C + 1 - H + X - N/2
The formula for degrees of unsaturation is a useful tool for determining the number of rings and multiple bonds in a compound. The formula is represented as:
> Unsat = C + 1 - (H + X - N/2)
In this formula, C represents the number of carbons, H is the number of hydrogens, X is the number of halogens, and N is the number of nitrogens. This formula is particularly applicable to organohalogen compounds (C, H, X) and organonitrogen compounds (C, H, N).
For organohalogen compounds, the halogen (X) substituent takes the place of a hydrogen atom in an organic molecule. As a result, we can determine the equivalent hydrocarbon formula by adding the number of halogens and hydrogens. For instance, the formula C4H6Br2 is equivalent to C4H8, indicating one degree of unsaturation.
On the other hand, oxygen atoms in organonitrogen compounds do not impact the formula of an equivalent hydrocarbon, and thus, they can be disregarded when calculating the degree of unsaturation. For example, the formula C5H8O corresponds to two degrees of unsaturation.
When dealing with organonitrogen compounds, it's important to remember that nitrogen forms three bonds, resulting in one additional hydrogen atom compared to a related hydrocarbon. Consequently, we subtract the number of nitrogens from the number of hydrogens to find the equivalent hydrocarbon formula. For instance, C5H9N is equivalent to C5H8, indicating two degrees of unsaturation.
By applying this formula, we can determine the degrees of unsaturation for various compounds and gain insights into their molecular structures, including the presence of rings and multiple bonds.
Now, for the specific case of C3H6O, we can use the formula to determine the degrees of unsaturation:
> Unsat = 3 + 1 - (6 + 0 - 0/2) = 3 + 1 - 6 = 4 - 6 = -2
So, for C3H6O, the calculation yields a negative value, indicating that it is not applicable in this context. However, it's important to note that the concept of constitutional isomers relates to molecules with the same molecular formula but different structural arrangements. In this case, there may be multiple constitutional isomers for C3H6O, but the formula for degrees of unsaturation may not be the most suitable tool for determining their specific structures.
The KKK's Constitution: A Twisted Interpretation
You may want to see also

The isomers can be conformational or constitutional
There are two main types of isomers: conformational isomers and constitutional isomers. Conformational isomers have different spatial arrangements of atoms within a molecule but maintain the same bond connectivity and configuration. These different arrangements arise from rotations around single bonds, leading to variations in dihedral angles between vicinal groups. For instance, ethane exhibits conformational isomerism with staggered, eclipsed, and skewed forms. On the other hand, constitutional isomers, also known as structural isomers, share the same molecular formula but exhibit different bonding patterns and atomic organization. An example is pentane, which has three constitutional isomers: n-pentane, isopentane, and neopentane, each with distinct carbon chain structures.
Constitutional isomers can be further categorized into skeletal isomers (chain isomers) and functional isomers. Skeletal isomers differ in the ordering of the skeleton or backbone of the molecule, often observed in organic compounds with long carbon chains. Functional isomers, meanwhile, vary in the way atoms are connected while sharing the same molecular formula. A notable example of functional isomerism is 1-hexene and cyclohexane. 1-hexene has a straight-chain structure with a carbon-carbon double bond, while cyclohexane has a cyclic structure without any carbon-carbon double bonds.
Conformational isomers, unlike constitutional isomers, generally cannot be separated at room temperature due to the ease of interconversion. The energy barriers for transitioning between conformational isomers are typically low, allowing for interconversions at room temperature. This is exemplified by the rapid interconversion of enantiomeric conformers X and X' in cis-1,2-dimethylcyclohexane, resulting in optical inactivity.
Additionally, conformational isomers can exhibit specific notations based on the dihedral angles between vicinal groups. Ethane, for instance, can exist in staggered (±60 degrees), eclipsed (0 degrees), or skewed (0 degrees < θ < 60 degrees) conformations. These conformational arrangements contribute to the overall stereoisomeric structure of the molecule. Conformational isomerism also plays a role in enzymatic synthesis and stereoselective chemical processes.
In summary, the concept of isomers encompasses both conformational and constitutional variations. Conformational isomers involve spatial arrangements of atoms within a molecule while sharing bond connectivity, as seen in different forms of ethane. Constitutional isomers, on the other hand, differ in their bonding patterns and atomic organization while retaining the same molecular formula, as illustrated by the isomers of pentane. Further classifications within constitutional isomers include skeletal isomers and functional isomers, each presenting unique structural characteristics. Understanding these isomeric forms is essential in fields such as organic chemistry and stereochemistry.
Strict Constructionism: Interpreting the Constitution Literally
You may want to see also
Frequently asked questions
There are 2 structural isomers possible for C3H6O.
The two isomers are those with molecular formulas of CH3COCH3 and CH3OCH2CH3.
The electron dot structures for CH3COCH3 and CH3OCH2CH3 are CH3•CO•CH3 and CH3•O•CH2CH3, respectively.
Constitutional isomers are isomers that have the same molecular formula but differ in the bonding of atoms and/or the arrangement of atoms in space.






















