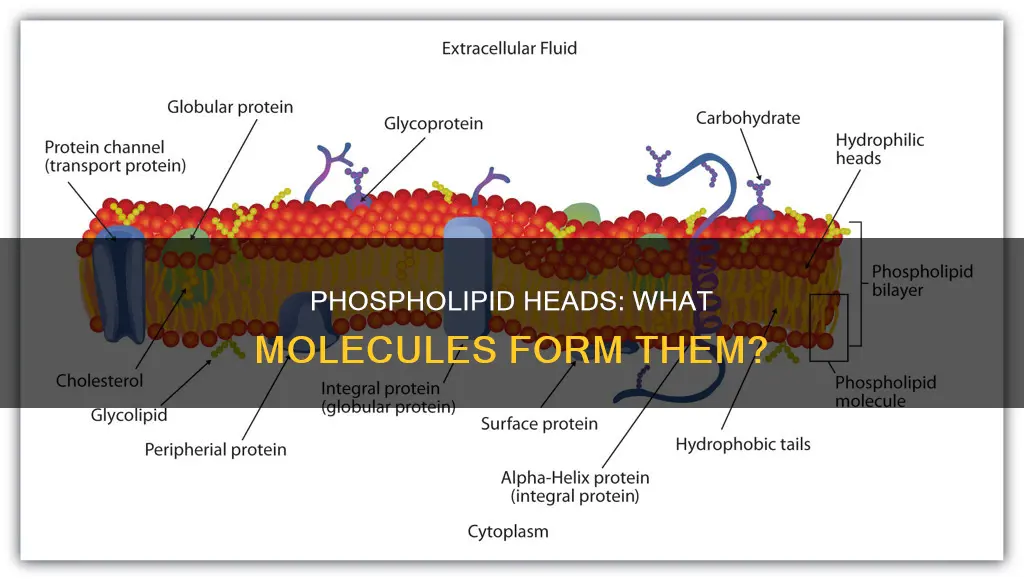
Phospholipids are a class of lipids that play a crucial role in cell membranes. They are composed of a hydrophilic head containing a phosphate group and two hydrophobic tails derived from fatty acids. The phosphate group, located at one end of the molecule, is negatively charged and hydrophilic, making it attracted to water. This hydrophilic head group consists of various combinations of functional groups, such as choline, ethanolamine, serine, or inositol, linked to the phosphate moiety. The fatty acid tails, on the other hand, are uncharged, nonpolar, and hydrophobic, causing them to be repelled by water. The unique structure of phospholipids, with their hydrophilic heads and hydrophobic tails, allows them to spontaneously form a lipid bilayer, a fundamental component of cell membranes.
| Characteristics | Values |
|---|---|
| Type of molecule | Phosphate group |
| Charge | Negative |
| Solubility | Hydrophilic |
| Structure | Polar |
| Composition | Phosphorus atom bonded to four oxygen atoms |
| Amphipathic | Yes |
| Head group type | Choline, ethanolamine, serine, inositol, glycerol |
Explore related products
What You'll Learn

Phospholipids are amphipathic molecules
The hydrophobic region of a phospholipid consists of two fatty acid "tails" derived from fatty acids. These tails are uncharged, nonpolar, and hydrophobic, causing them to be repelled by water. The fatty acid chains can be saturated, containing single bonds between carbon atoms, or unsaturated, containing one or more double bonds. The composition of the fatty acid tails determines the physical properties of the phospholipid.
When placed in water, phospholipids spontaneously form a structure known as a micelle, with their hydrophilic heads oriented toward the water and their hydrophobic tails pointing inward away from the water. This arrangement allows phospholipids to form a lipid bilayer, which is the structural basis of all cell membranes. The lipid bilayer acts as a selective barrier, regulating the movement of substances into and out of the cell.
The amphipathic nature of phospholipids gives them unique properties that are essential for the structure and function of cell membranes. The hydrophilic heads face outward on each surface of the bilayer, interacting with the surrounding aqueous environment, while the hydrophobic tails are shielded from the water in the interior, forming a hydrophobic core. This configuration allows for the controlled entry and exit of chemicals and helps maintain the integrity of the cell membrane.
Understanding Civil Penalty Patterns in Electrical Safety
You may want to see also

The head group is hydrophilic
Phospholipids are a class of lipids with a hydrophilic head and a hydrophobic tail. The head of a phospholipid molecule is hydrophilic because it contains a phosphate group that is attracted to water. This phosphate group is negatively charged, making it polar and hydrophilic. The phosphate group is linked to a hydrophilic head group, which determines the specific type of phospholipid.
The head group is composed of various combinations of functional groups, such as choline, ethanolamine, serine, or inositol, linked to the phosphate moiety. These functional groups contribute to the hydrophilic nature of the head group.
The hydrophilic nature of the head group is important for the function of phospholipids in biological membranes. When placed in water, phospholipids spontaneously form structures called micelles, with their hydrophilic heads oriented toward the water. This arrangement allows the hydrophilic heads to interact with the aqueous environment, while the hydrophobic tails are shielded from the water and aggregated together.
The hydrophilic heads of phospholipids play a crucial role in maintaining the stability and function of biological membranes. The polar and charged nature of the head group allows it to interact with water molecules and other polar molecules in the surrounding environment. This interaction helps to stabilize the membrane structure and facilitates the transport of substances through the membrane.
Furthermore, the hydrophilic head group also contributes to the selective permeability of the membrane. The phospholipid bilayer, formed by the aggregation of phospholipids, acts as a selective barrier that regulates the movement of substances into and out of the cell. The hydrophilic heads form the outer surface of the bilayer, while the hydrophobic tails face inward, creating a hydrophobic core. This arrangement allows hydrophilic molecules and ions to pass through the membrane more easily, while hydrophobic molecules interact with the hydrophobic core, facilitating their transport across the membrane.
Psychotherapy Notes vs Medical Records: Understanding the Distinction
You may want to see also

Phospholipids form bilayers
Phospholipids are a class of lipids with a hydrophilic "head" and two hydrophobic "tails". The head contains a phosphate group, while the tails are derived from fatty acids and are joined by an alcohol residue, usually a glycerol molecule.
The hydrophobic tails, on the other hand, are repelled by water and thus aggregate to minimise contact with it. This results in the formation of a membrane with a hydrophilic surface and a hydrophobic core. The specific properties of phospholipids allow them to play a crucial role in the cell membrane, where they contribute to its fluidity and flexibility.
The phospholipid bilayer acts as a selective barrier, regulating the movement of substances into and out of the cell. It separates the internal contents of the cell from its surroundings, allowing cells to maintain their internal conditions and control the transport of essential molecules. The hydrophobic core of the bilayer restricts the passage of hydrophilic molecules and ions, while small hydrophobic molecules can diffuse through the lipid tails. This selective permeability is essential for biological functions such as cell communication and metabolism.
The phospholipid bilayer is a fundamental structure in the creation of a living cell, providing structural integrity and dynamic functionality to cell membranes. It is also utilised in various applications, such as drug delivery systems, where its unique properties are harnessed to improve bioavailability and reduce toxicity.
Federal Regulations: Constitutionality of Title 8 CFR
You may want to see also
Explore related products

The phosphate group is negatively charged
Phospholipids are a class of lipids with a hydrophilic "head" and a hydrophobic "tail". The hydrophilic head contains a phosphate group, which is negatively charged. This negative charge is due to the presence of a phosphorus atom bonded to four oxygen atoms in the phosphate group. One of these oxygen atoms is also bonded to the glycerol backbone of the phospholipid molecule.
The negative charge of the phosphate group is important for the function of phospholipids in biological membranes. Due to its negative charge, the phosphate head is polar and hydrophilic, meaning it is attracted to water molecules in its environment. On the other hand, the lipid tails are uncharged, nonpolar, and hydrophobic, meaning they repel water. This results in the spontaneous formation of a phospholipid bilayer when phospholipids are placed in water. The bilayer consists of two layers of phospholipids, with their heads exposed to the water on both sides and their tails directed into the membrane, forming a hydrophobic core.
The phospholipid bilayer acts as a selective barrier that regulates the movement of substances into and out of cells. The hydrophobic core of the bilayer restricts the passage of hydrophilic molecules and ions, while small hydrophobic molecules can diffuse through the lipid tails. This selective permeability is essential for maintaining the internal conditions of cells and controlling the transport of essential molecules.
The negative charge of the phosphate group also contributes to the fluidity and flexibility of biological membranes. The phosphate heads are in constant motion due to their attraction to water molecules, which adds to the overall fluidity of the membrane. Additionally, the unsaturated hydrophobic tails prevent the phospholipid molecules from packing too closely together, further contributing to the fluidity and dynamics of the membrane.
The specific type of phospholipid is determined by the hydrophilic head group, which can consist of various combinations of functional groups, such as choline, ethanolamine, serine, or inositol, linked to the phosphate moiety. The phosphate group can also be modified with simple organic molecules, further influencing the properties and functions of phospholipids in biological systems.
Checks and Balances: The Constitution's Core Principle
You may want to see also

The head group determines the phospholipid type
Phospholipids are a class of lipids composed of a hydrophilic head group, a glycerol molecule, and two hydrophobic fatty acid tails. The head group is a phosphate group that is hydrophilic because it carries an electric charge, attracting it to water. The fatty acid chains form the hydrophobic tails, which are repelled by water. This combination of hydrophilic and hydrophobic groups makes phospholipids amphipathic molecules.
The head group determines the type of phospholipid. The hydrophilic head group consists of various combinations of functional groups, such as choline, ethanolamine, serine, inositol, or glycerol, linked to the phosphate moiety. These head groups can differ in size, shape, and charge. For example, phosphatidylcholine, phosphatidylethanolamine, phosphatidylserine, and sphingomyelin are four major phospholipids in mammalian cell membranes, each with different head groups.
The specific type of phospholipid is important because it determines its function. For instance, inositol phospholipids are present in smaller quantities but are very important for cell signaling. Some membrane proteins can only function in the presence of specific phospholipid head groups, just as enzymes in an aqueous solution require a particular ion for activity. The head group also determines the phospholipid's interaction with water and its overall polarity.
Phospholipids play a crucial role in cell membranes, with their hydrophilic head groups interacting with the surrounding aqueous environment while facing outward. The hydrophobic fatty acid tails face inward, forming a hydrophobic core. This arrangement allows phospholipids to spontaneously form a lipid bilayer, which acts as a selective barrier regulating the movement of substances into and out of the cell.
The phospholipid bilayer consists of two adjacent sheets of phospholipids, arranged tail to tail, with the hydrophilic heads exposed to the liquid on both sides and the hydrophobic tails directed into the membrane. This structure provides structural integrity to cell membranes and allows for membrane dynamics and cell movements.
Understanding TurboTax's "Other Income and Loss" Section
You may want to see also
Frequently asked questions
The head of a phospholipid is a hydrophilic molecule, meaning it is attracted to water. The head consists of a phosphate group and can be modified with simple organic molecules such as choline, ethanolamine, serine, or inositol.
The hydrophilic head is attracted to the intracellular and extracellular fluid, allowing it to interact with the surrounding aqueous environment. This arrangement provides structural integrity to cell membranes.
The molecule at the opposite end of the hydrophilic head is the fatty acid tail, which is hydrophobic and water-insoluble.