Constitutional isomers, also known as structural isomers, are molecules with the same molecular formula but different bonding arrangements. They have different connectivities of atoms, meaning the atoms are connected differently despite having the same ratio of atoms. For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers of the molecule C2H6O as they have different atom connections but the same atoms in the same ratios. Positional isomers, a type of constitutional isomer, have the same functional groups but differ in their location within the molecule. Stereoisomers, another type of isomer, have the same connectivity but differ in the orientation of their constituent atoms in space. To identify constitutional isomers, one can use the HDI formula, which corresponds to the number of H2 equivalents required to saturate a molecule to have only open-chain single-bond structures.
| Characteristics | Values |
|---|---|
| Molecular formula | Same |
| Connectivity of atoms | Different |
| Structural formulas | Different |
| Bonding arrangements | Different |
| Functional groups | Same or different |
| Location of functional groups | Different |
| HDI indexes | Same |
Explore related products
$17.99 $19.99
What You'll Learn
- Constitutional isomers have the same molecular formula but different connectivity
- They have the same functional groups but differ in their location
- Stereoisomers have the same connectivity but differ in the arrangement of atoms
- HDI indexes can be used to draw constitutional isomers
- They can be identified by their linear structures

Constitutional isomers have the same molecular formula but different connectivity
Constitutional isomers are molecules that share the same molecular formula but differ in their atomic connectivity. In other words, constitutional isomers have the same types and numbers of atoms but differ in the way these atoms are bonded or connected to one another. This means that constitutional isomers have distinct chemical structures and, consequently, distinct chemical properties.
For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers with the molecular formula C2H6O. Despite sharing the same atoms in the same ratios, these two molecules differ in the way the atoms are connected. As a result, ethanol and dimethyl ether are considered distinct molecules with distinct chemical properties.
The concept of constitutional isomers is closely related to the idea of structural isomers. Structural isomers are compounds that have the same molecular formula but differ in the arrangement of their atoms or groups of atoms. This means that structural isomers can be thought of as a type of constitutional isomer, where the connectivity of atoms is specifically defined by the arrangement of atomic groups.
The identification and analysis of constitutional isomers are important in various fields, including organic chemistry and biochemistry. For instance, in organic chemistry, understanding constitutional isomers can help predict the properties and behaviours of different molecules. Additionally, in biochemistry, the study of constitutional isomers can provide insights into the structure and function of complex biomolecules, such as proteins and nucleic acids.
Furthermore, the concept of constitutional isomers is closely tied to the idea of isomers in general. Isomers are molecules that share the same molecular formula but differ in their atomic arrangement or connectivity. This means that constitutional isomers are a specific type of isomer, where the focus is on the distinct connectivity of atoms within the molecule. Other types of isomers include stereoisomers, which have the same connectivity but differ in the spatial arrangement of atoms or groups of atoms.
Identifying Non-Spill Factors: What Doesn't Count as Spillage?
You may want to see also

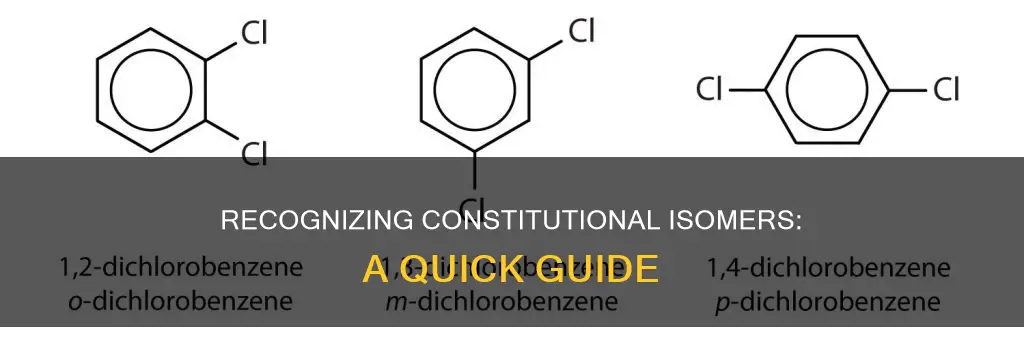
They have the same functional groups but differ in their location
Constitutional isomers are molecules that have the same molecular formula but differ in the way their atoms are connected. In other words, they have the same types and numbers of atoms but differ in the way these atoms are bonded together. This means that constitutional isomers have the same functional groups but differ in the positioning of these groups within the molecule.
A functional group is a specific group of atoms or bonds within a compound that is responsible for the compound's characteristic chemical reactions. In organic chemistry, a functional group is any substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables the systematic prediction of chemical reactions and the behavior of chemical compounds, as well as the design of chemical synthesis.
Functional groups can be used to "functionalize" a compound, giving it different physical and chemical properties than it would have in its original form. They can also be used to distinguish similar compounds from each other. For example, the amide functional group has the formula R-(CO)-NR2 and has a carbonyl carbon bonded to a nitrogen atom, which is in turn bonded to two other alkyl groups. Other common functional groups include hydroxyl, ketone, amine, ether, and haloalkanes or alkyl halides.
Constitutional isomers, as a result of having the same functional groups but different connectivity, will exhibit different properties due to the different arrangements of these functional groups. For example, 1-propanol and 2-propanol are isomers that have a hydroxyl group on different carbon atoms. This difference in the location of the functional group within the molecule results in distinct molecular properties.
Shays' Rebellion: Constitution's Testing Ground
You may want to see also

Stereoisomers have the same connectivity but differ in the arrangement of atoms
Constitutional isomers have the same molecular formula but differ in the way atoms are connected. For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers with the molecular formula C2H6O. However, the connections between those atoms differ.
Now, onto stereoisomers. Stereoisomers have the same connectivity but differ in the arrangement of atoms in space. They can be further divided into configurational stereoisomers and conformational stereoisomers. The precise specification of the spatial arrangement of the groups in a configurational isomer is called its configuration, and in a conformational isomer, it is called its conformation.
Stereoisomers cannot be superimposed on each other by any rotations about single bonds. To make them superimposable, rotation about a double bond or dissociation of one or more single bonds, or both, is necessary. These processes usually require a lot of energy and do not occur at room temperature.
An example of stereoisomers is 3-methyl-1-pentene, which arises from tetrahedral carbon atoms attached to four different substituents. There are two different ways to arrange the four different groups around a tetrahedral center, resulting in two molecules with the same connectivity but a different arrangement of atoms. These molecules are non-superimposable mirror images of each other.
Another way to think about stereoisomers is through the concept of "handedness" in molecules, similar to the left-hand and right-hand relationship. If you place both palms facing up and next to each other, and then flip one hand over to the other, one hand will show the back of the hand, while the other will show the palm. They are not the same and are non-superimposable, just like stereoisomers.
George W. Bush: Constitutional Violator in Chief
You may want to see also
Explore related products
$42.23 $49.99

HDI indexes can be used to draw constitutional isomers
Constitutional isomers are molecules with the same molecular formula but different atomic connectivity. They have different properties and can be identified by counting the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index, or HDI). If the molecules have the same number of atoms and the same HDI, they are constitutional isomers.
The HDI is calculated using a simple formula and is a very useful tool, especially when drawing constitutional isomers for different molecules. The HDI value is derived from the combination of cycles and double or triple bonds in a molecule. Since constitutional isomers have the same molecular formula, they will also have the same HDI indexes. Therefore, knowing the HDI of a molecule allows for the automatic drawing of various constitutional isomers with the correct structural motifs.
For example, let's consider a molecule with the molecular formula C3H6O. Based on an HDI of 1, we know that this molecule must contain either a double bond or a cycle, but not both. We can then draw the possible linear structures for the constitutional isomers, adding the necessary number of hydrogens while being careful to maintain consistent bonding patterns.
Finally, we can explore any potential branches from the core atoms and investigate the possibility of cyclic structures. By following this approach, we can efficiently explore the different structural possibilities for constitutional isomers.
Harassment for Money: Understanding Your Rights on Phone Calls
You may want to see also

They can be identified by their linear structures
Constitutional isomers are molecules with the same molecular formula but different structural formulas or bonding arrangements. They have different connectivities of atoms, meaning that the atoms are connected differently even though the ratio of atoms remains the same. For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers of the molecule C2H6O. While they have the same atoms in the same ratios, the connections between those atoms differ.
When identifying constitutional isomers, it is helpful to start by considering the possible linear structures. This involves first determining the core structure and then adding the necessary number of hydrogens while being careful not to add too many and maintaining consistency with typical bonding patterns in organic molecules. By focusing on the linear structures, we can identify the potential constitutional isomers by examining the different ways in which the atoms can be connected while maintaining the same molecular formula.
For instance, let's consider the molecule C2H6O again. We can start by drawing the linear structure, ensuring we have the correct number of atoms and maintaining standard bonding patterns. From there, we can explore different arrangements of atoms while keeping the same molecular formula. This exploration of various linear structures allows us to identify potential constitutional isomers.
In addition to linear structures, constitutional isomers can also be recognised by their branching patterns. After establishing the core structure, we can explore the possibility of adding branches to the side, creating more complex arrangements. These branches introduce variations in the connectivity of atoms, further differentiating the isomers.
Furthermore, constitutional isomers can be identified by their cyclic structures. Cyclic motifs play a role in the molecule's HDI (Hydrogen-Deuterium Exchange Index) value, which is related to the degree of unsaturation. By considering the combination of cycles and double or triple bonds, we can determine the HDI value. Since constitutional isomers have the same molecular formula, they will also share the same HDI values, providing another method for identification.
In summary, when identifying constitutional isomers, a systematic approach can be employed. Begin by drawing the possible linear structures, ensuring adherence to bonding patterns and atom counts. Then, explore branching options and cyclic structures to further differentiate the isomers. Finally, consider the HDI values, which will be consistent among constitutional isomers due to their identical molecular formulas. By following these steps, we can effectively identify constitutional isomers through their linear, branched, and cyclic structures, all while maintaining the same molecular formula.
The Preamble: Constitution's Justification and Explanation
You may want to see also
Frequently asked questions
Constitutional isomers are molecules that have the same molecular formula but differ in their connectivity, or the way in which the constituent atoms are connected to one another.
To identify constitutional isomers, you should first determine if the molecules have the same molecular formula. Then, examine if they have different structural formulas or bonding arrangements. Finally, look for differences in the connectivity of atoms within the molecules.
Ethanol (ethyl alcohol) and dimethyl ether are examples of constitutional isomers. They have the same molecular formula, C2H6O, but differ in the connectivity of their atoms. Another example is the pair of isomers 1-propanol and 2-propanol, which have a hydroxyl group on different carbon atoms.
Constitutional isomers differ from stereoisomers, which have the same connectivity of atoms but differ in the spatial arrangement of their constituent atoms. Stereoisomers can be further classified into configurational stereoisomers and conformational stereoisomers. Other types of isomers include geometric isomers, optical isomers, and regioisomers.




















![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 100 Milligrams](https://m.media-amazon.com/images/I/71-dKlpNLrS._AC_UL320_.jpg)

![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 250 Milligrams](https://m.media-amazon.com/images/I/71EL45ccGFS._AC_UL320_.jpg)
![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 50 Milligrams](https://m.media-amazon.com/images/I/81HAPnASH5L._AC_UL320_.jpg)



















