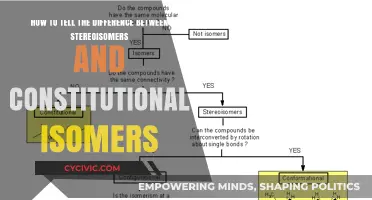
Constitutional isomers and geometric isomers are types of stereoisomers, which are molecules with the same molecular formula but different structural connectivity. The key difference between constitutional and geometric isomers lies in their structural characteristics and how they are identified. Constitutional isomers are identified by counting the number of carbons and the degree of unsaturation, while geometric isomers are distinguished by their spatial arrangement around a double bond, resulting in different chemical properties. Understanding these differences is crucial in fields such as organic chemistry, where the unique properties of isomers play a significant role in synthesis and reactions.
| Characteristics | Values |
|---|---|
| Molecular Formula | Same |
| Atomic Composition | Same |
| Atomic Connectivity | Different |
| Atomic Arrangement | Different |
| Bonding Patterns | Different |
| Structure | Different |
| Index of Hydrogen Deficiency (IHD) | Same |
Explore related products
$19.65
What You'll Learn

Constitutional isomers have the same molecular formula
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivity. They have the same types and numbers of atoms but differ in how these atoms are structurally connected. For example, butane (C4H10) can have different structures where the four carbons and ten hydrogens are connected differently, making them constitutional isomers. Another example is the formula C2H6O, which corresponds to both ethanol (ethyl alcohol) and dimethyl ether. These isomers have the same molecular mass but different physical and chemical properties due to their distinct atomic connections.
To identify constitutional isomers, the first step is to determine the molecular formula of the compound. This involves counting the number of carbons, heteroatoms, and hydrogens present. By understanding that carbon must have four bonds, we can calculate the number of hydrogens by subtracting the number of bonds at each carbon from four. Once the molecular formula is established, we can compare it to other compounds with the same formula but different structures.
The Index of Hydrogen Deficiency (IHD) or the Hydrogen Deficiency Index (HDI) is a valuable tool for identifying constitutional isomers. By calculating the HDI, we can determine the presence of double bonds or rings in the molecule. For instance, an IHD of 0 indicates the absence of double bonds or rings, while an IHD of 1 suggests the possibility of either a double bond or a ring. The HDI helps us draw various constitutional isomers with the correct structural motifs.
Additionally, it is important to name the molecules according to the IUPAC nomenclature rules, especially for large molecules, to ensure accuracy in identifying constitutional isomers. While constitutional isomers have the same molecular formula, their distinct atomic connections lead to different properties. These differences can impact the stability, naming conventions, melting and boiling points, and interactions with other compounds. Therefore, recognizing constitutional isomers is crucial in understanding the unique characteristics of each isomer.
Gaining Senate Majority: The Magic Number of Seats
You may want to see also

They have different bonding patterns
Constitutional isomers, also known as structural isomers, are organic compounds that have the same molecular formula but differ in their bonding patterns and atomic organisation. They have different connectivity of atoms within the molecules.
To identify constitutional isomers, the first step is to create a molecular formula for the compound. This formula will show the composition of the molecule, i.e., the types and numbers of atoms present, but it will not reveal how these atoms are connected. For example, butane (C4H10) can have multiple structures that satisfy its chemical formula. While all these structures have four carbons and ten hydrogens, they are connected differently and are therefore constitutional isomers. Another example is ethanol (ethyl alcohol) and dimethyl ether, which are constitutional isomers as they have the same types and ratios of atoms but differ in the connections between those atoms.
Constitutional isomers can also be identified using the Index of Hydrogen Deficiency (IHD). Since constitutional isomers have the same molecular formula, they will have the same HDI indexes. The combination of cycles, double bonds, and triple bonds in a molecule will give its HDI value. Knowing the HDI of a molecule allows for the drawing of various constitutional isomers with the correct structural motifs.
Chain isomers, or skeletal isomers, are constitutional isomers in which the components of the molecule's skeleton are ordered differently, resulting in distinct skeletal structures. This type of isomerism is commonly observed in organic compounds with long carbon chains. Functional isomers are constitutional isomers that share the same molecular formula but differ in how their atoms are connected. An example of functional isomerism is 1-hexene and cyclohexane.
The Constitution and Federal Courts: Explicit or Implied?
You may want to see also

They have different connectivity of atoms
Constitutional isomers, also known as structural isomers, are organic compounds that have the same molecular formula but differ in their bonding atomic organisation and bonding patterns. This means that they have the same number and type of atoms but differ in how these atoms are connected. For example, butane (C4H10) can have several structures where the four carbons and ten hydrogens are connected differently, making them constitutional isomers.
To identify constitutional isomers, the first step is to create a molecular formula for the compound. This formula tells us the composition of the molecule, i.e., what atoms are present and how many there are, but it does not reveal how the atoms are connected. For instance, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers as they have the same atoms in the same ratios, but the connections between those atoms differ.
The Index of Hydrogen Deficiency (IHD) is a useful tool for identifying constitutional isomers. Since constitutional isomers have the same molecular formula, they will also have the same IHD indexes. By knowing the IHD for a molecule, you can draw various constitutional isomers with the correct structural motifs. The combination of cycles, double bonds, and triple bonds in a molecule will give you its IHD value.
Constitutional isomers can also be categorised as chain isomers or skeletal isomers, where the components of the molecule's skeleton are ordered differently, resulting in distinct skeletal structures. This type of isomerism is commonly found in organic compounds with long carbon chains. Additionally, functional isomers are constitutional isomers that share the same molecular formula but differ in how the atoms are connected. An example of functional isomerism is observed in 1-hexene and cyclohexane.
Addressing Constitutional Issues: Individual Rights and Freedoms
You may want to see also
Explore related products

They have different structures
Constitutional isomers, also known as structural isomers, are organic compounds that have the same molecular formula but differ in their structural connectivity. This means that the molecules have the same number and type of atoms but differ in how these atoms are bonded and arranged in 3D space.
For example, let's consider the molecules ethanol (ethyl alcohol) and dimethyl ether. Both of these molecules have the same molecular formula, C2H6O, but they differ in the way their atoms are connected. In ethanol, the carbon and oxygen atoms are directly bonded to each other, while in dimethyl ether, the carbon and oxygen atoms are bonded through a single bond. This difference in atomic connectivity distinguishes these molecules as constitutional isomers.
Another example is butane, which can have multiple structural isomers. Butane is represented by the chemical formula C4H10, indicating that it contains four carbon atoms and ten hydrogen atoms. However, the arrangement of these atoms can vary, resulting in different structural isomers. For instance, in one isomer, the carbon atoms may form a straight chain, while in another isomer, the carbon chain may be branched.
Constitutional isomers can also arise from differences in the arrangement of cycles, double bonds, or triple bonds within a molecule. For instance, 1-hexene and cyclohexane are constitutional isomers as they share the same molecular formula but differ in their bonding patterns. The presence of a double bond in 1-hexene and a cyclic structure in cyclohexane highlights the variation in their atomic connections.
Identifying constitutional isomers often involves creating a molecular formula for the compound and then exploring different ways to connect the atoms while maintaining the same formula. This can be facilitated by using tools such as the Index of Hydrogen Deficiency (IHD) or Lewis structures to determine the potential structural arrangements that satisfy the given molecular formula.
Lindy Boggs: Hale's Constitution Successor
You may want to see also

They can be identified using the Index of Hydrogen Deficiency
Constitutional isomers are organic compounds with the same molecular formula but a different 3D arrangement of atoms. They differ in how they are structurally connected. For example, butane (C4H10) can have several structures that satisfy its chemical formula. While both structures have four carbons and ten hydrogens, they are connected differently and are therefore constitutional isomers.
To identify constitutional isomers, you can use the Index of Hydrogen Deficiency (IHD). The IHD is a measure of the number of hydrogen pairs missing from a molecule compared to a fully saturated hydrocarbon. It helps determine the degree of unsaturation (double bonds, rings, etc.).
To calculate the IHD, you can use the following equation:
IHD = 2 x (number of carbon atoms) + (number of nitrogen atoms) + 2 - (number of hydrogen and halogen atoms)
If the IHD values and non-hydrogen atoms of two compounds are identical, you can proceed to check the connectivity of the atoms. If the connections are the same, the compounds are identical. If the connections differ, they are constitutional isomers.
For example, if you have a compound with the formula C4H7NO, you can calculate its degree of unsaturation as 2. This means that this compound is missing four hydrogens, which helps identify the atoms present in the compound.
By using the IHD, you can determine the number of rings or double bonds possible in a structure. For instance, an IHD of 0 indicates that there will be no rings or double bonds, while an IHD of 1 means that there can be either a double bond or a ring.
Framers' Foresight: Constitution's Adaptive Nature
You may want to see also
Frequently asked questions
First, create a molecular formula for the compound by counting the number of carbons, heteroatoms, and hydrogens present. Then, compare the degree of unsaturation using the Hydrogen Deficiency Index (HDI). If all the atoms are the same and the molecules have the same HDI, they are constitutional isomers.
Constitutional isomers have the same molecular formula but differ in how their atoms are structurally connected. Geometric isomers, on the other hand, have the same molecular formula and connectivity but differ in the arrangement of their atoms in space.
Butane (C4H10) can have different structures, making it a constitutional isomer. Another example is the formula C2H6O, which can represent ethanol (ethyl alcohol) or dimethyl ether, which have different connections between their atoms.
The connectivity of atoms in constitutional isomers influences their properties. It can impact a compound's stability, naming, melting point, and boiling point.