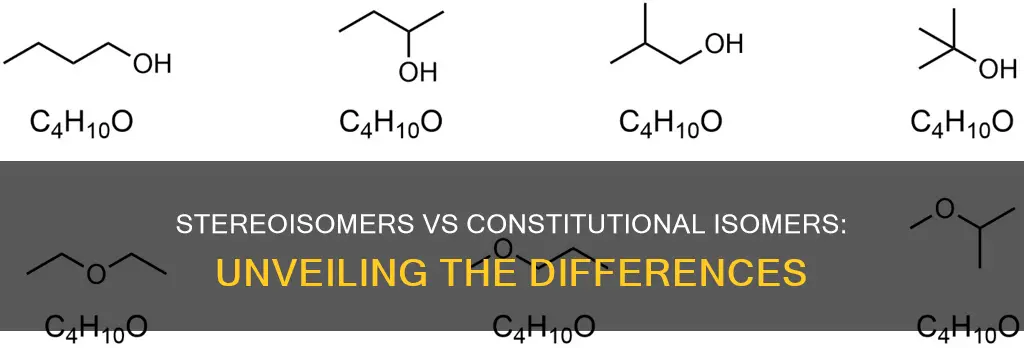
Isomers are compounds that share the same molecular formula but differ in structure. There are two main types of isomers: constitutional isomers and stereoisomers. Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of their atoms. Stereoisomers, on the other hand, have the same connectivity but differ in their spatial arrangement, resulting in distinct molecules. This distinction is crucial in organic chemistry, particularly when analyzing molecular relationships and determining structural variations.
| Characteristics | Constitutional Isomers | Stereoisomers |
|---|---|---|
| Molecular Formula | Same | Same |
| Connectivity | Different | Same |
| Spatial Arrangement | Different | Different |
| Types | Skeletal isomers, Positional isomers | Cis and trans isomers, Enantiomers, Diastereomers |
| Examples | Butane and isobutane | Cis-trans isomers, Enantiomers |
Explore related products
$17.99 $19.99
What You'll Learn
- Constitutional isomers have the same molecular formula but different connectivity
- Stereoisomers have the same connectivity but different shapes
- Cis and trans isomers are types of stereoisomers with different substituent arrangements
- Constitutional isomers have different structures and can be skeletal or positional isomers
- Stereoisomers are always different molecules, unlike conformers

Constitutional isomers have the same molecular formula but different connectivity
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in their connectivity. This means that the atoms are connected in different ways, resulting in distinct structures. For example, butane (C4H10) and isobutane (C4H10) share the same molecular formula but differ structurally. Butane has a straight chain, while isobutane exhibits a branched chain. This disparity in connectivity categorises them as constitutional isomers.
Constitutional isomers can be further classified into three subtypes: skeletal isomers, positional isomers, and functional isomers. Skeletal isomers maintain a uniform quantitative, qualitative, and functional composition but exhibit a structurally distinct chain of molecules. These chains may be straight or exhibit varying degrees of branching. Positional isomers, on the other hand, differ in the location of a functional group, substituent, or complex bonds within the chain. The primary distinction between these subtypes lies in their physical properties, with some positional isomers also differing in their biochemical characteristics.
The concept of constitutional isomers is particularly relevant in organic chemistry, where molecules with identical chemical formulas can exhibit different connections. This understanding is crucial for analysing molecular relationships and predicting structural variations. For instance, the formula C8H18 can be represented in multiple ways, giving rise to structural isomers with potentially diverse properties.
It is important to distinguish constitutional isomers from stereoisomers. While constitutional isomers vary in their connectivity, stereoisomers share the same connectivity but differ in their spatial arrangement. Stereoisomers include enantiomers and diastereomers, which are mirror images that cannot be superimposed. Stereoisomers play a significant role in biology, as the molecules used by the body to build proteins must all be the same isomer.
Swearing to Serve: National Guard's Oath to the Constitution
You may want to see also

Stereoisomers have the same connectivity but different shapes
Isomers are compounds that have the same molecular formula but different structures. There are two main types of isomers: constitutional isomers and stereoisomers. Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the connectivity of their atoms. On the other hand, stereoisomers have the same connectivity but different shapes. This means that the atoms are connected in the same way but arranged differently in space, resulting in distinct structures. Stereoisomers are always different molecules because they are stuck in their respective shapes and cannot be interconverted.
Stereoisomers can be further classified into two types: enantiomers and diastereomers. Enantiomers are stereoisomers that are non-superimposable mirror images of each other. They have the same molecular formula and connectivity but differ in how their atoms are arranged in space. Diastereomers, on the other hand, are stereoisomers that are not mirror images. They also share the same molecular formula and connectivity but differ in their spatial arrangement. Examples of stereoisomers include cis-trans isomers, where the substituents are arranged differently around a double bond or a ring. In cis isomers, the substituents are on the same side, while in trans isomers, they are on opposite sides.
The distinction between constitutional isomers and stereoisomers is crucial in organic chemistry, especially when analyzing molecular relationships. The concept of index of hydrogen deficiency (IHD) helps determine saturation and structural variations within these isomeric forms. Additionally, the specific case of stereoisomers is biologically significant because the molecules used by the body to build proteins must all be the same isomer. Stereoisomers can exhibit different physical and chemical properties due to their unique spatial arrangements, making them functionally distinct from one another.
To identify stereoisomers, one must first ensure that the molecules share the same molecular formula. Then, the connectivity of the atoms should be examined. If the connectivity is the same but the spatial arrangement differs, resulting in distinct shapes, the molecules are stereoisomers. It is important to note that stereoisomers cannot be interconverted between their respective shapes, reinforcing their distinct nature. This is in contrast to conformers, which are essentially the same molecule but with a single bond rotated.
Challenging a Statute: Pennsylvania's Constitutional Process
You may want to see also

Cis and trans isomers are types of stereoisomers with different substituent arrangements
Isomers are molecules with the same chemical formula but different structures. They are classified into different types based on their molecular formula, connectivity, and shape. Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the connectivity of their atoms. This means that the atoms are connected in different ways, leading to different structures.
Stereoisomers, on the other hand, have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. This means that stereoisomers are different molecules that cannot be interconverted between one another. They are stuck in their respective shapes. An example of a stereoisomer is cis-trans isomerism, where the isomers have different configurations due to the presence of a rigid structure in their molecule.
Cis and trans isomers are types of stereoisomers that differ in the arrangement of substituents around a double bond or a ring. In the context of chemistry, "cis" indicates that the substituents are on the same side of some plane, while "trans" indicates that they are on opposite sides. For example, in 2-butene, the cis isomer has both methyl groups on the same side of the double bond, while the trans isomer has them on opposite sides.
The difference in spatial arrangement between cis and trans isomers can lead to distinct physical and chemical properties. For instance, trans isomers tend to have lower densities and higher melting points than cis isomers. The difference in stability between cis and trans isomers can be attributed to the unfavorable steric interaction of the substituents in the cis isomer.
Florida Diploma: Constitution Test Requirement Explained
You may want to see also
Explore related products
$42.23 $49.99

Constitutional isomers have different structures and can be skeletal or positional isomers
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the connectivity of their atoms. This means that the atoms are connected in different ways, leading to different structures. For example, butane (C4H10) and isobutane (C4H10) have the same molecular formula but different structures. Butane has a straight chain, while isobutane has a branched chain. This difference in connectivity classifies them as constitutional isomers.
Constitutional isomers can be further classified into skeletal isomers and positional isomers. Skeletal isomers differ in the atoms and bonds that are considered to comprise the "skeleton" of the molecule. For organic compounds, such as alkanes, this typically refers to the carbon atoms and the bonds between them. For instance, n-pentane, isopentane, and neopentane are skeletal isomers of pentane.
Positional isomerism, also known as regioisomerism, occurs when a functional group can occupy different positions on the same carbon chain. In simpler terms, positional isomers differ only in the position of a functional group, substituent, or some other feature on the same "parent" structure. For example, replacing one of the hydrogen atoms in n-pentane with a hydroxyl group can result in three different positional isomers.
It is important to note that stereoisomers and constitutional isomers are distinct concepts. While constitutional isomers differ in connectivity, stereoisomers have the same connectivity but differ in their spatial arrangement. Stereoisomers are always different molecules and cannot be interconverted, whereas constitutional isomers can have the same parts but are arranged differently.
The Constitution and Marriage: Man and Woman?
You may want to see also

Stereoisomers are always different molecules, unlike conformers
Isomers are molecules that share the same molecular formula but differ in structure. They are classified into different types based on their molecular formula, connectivity, and shape. Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the connectivity of their atoms. Stereoisomers, on the other hand, have the same connectivity but different shapes.
Conformers are a type of constitutional isomer. They are essentially the same molecule but differ in the rotation about a single bond. Stereoisomers, unlike conformers, are always different molecules. They differ in the spatial arrangement of their atoms, which can lead to different physical and chemical properties. For example, cis-trans isomers are stereoisomers that differ in the arrangement of substituents around a double bond or a ring. In cis isomers, the substituents are on the same side, while in trans isomers, they are on opposite sides.
The distinction between stereoisomers and conformers is important in organic chemistry. Stereoisomers cannot be interconverted between one another, as they are stuck in their respective shapes. This is because stereoisomers have different spatial arrangements of atoms, while conformers only differ in the rotation of a single bond. Therefore, stereoisomers and conformers represent distinct types of isomers with different molecular properties.
Additionally, stereoisomers can be further classified into enantiomers and diastereomers. Enantiomers are stereoisomers that are non-superimposable mirror images, while diastereomers are stereoisomers that are not mirror images. This distinction is similar to the relationship between identical and non-identical twins, where enantiomers are like identical twins, and diastereomers are like non-identical twins.
In summary, stereoisomers are always different molecules, unlike conformers, which are essentially the same molecule with a different rotation of a single bond. Stereoisomers have different spatial arrangements of atoms, leading to distinct physical and chemical properties. This distinction is crucial in understanding molecular relationships, especially in the context of organic chemistry and biological processes.
Weed Prohibition: Unconstitutional?
You may want to see also
Frequently asked questions
Stereoisomers have the same molecular formula and connectivity but differ in their spatial arrangement. This means that stereoisomers are always different molecules that cannot be interconverted. Examples of stereoisomers include cis-trans isomers and enantiomers. Cis isomers have their substituents on the same side, while trans isomers have them on opposite sides.
Constitutional isomers share the same molecular formula but differ in the connectivity of their atoms. This means that the atoms are connected in different ways, leading to different structures. For example, butane (C4H10) and isobutane (C4H10) have the same molecular formula but different structures, with butane having a straight chain and isobutane having a branched chain.
No, a molecule cannot be both a stereoisomer and a constitutional isomer. While a molecule can be several types of isomers at the same time, it cannot be both a stereoisomer and a constitutional isomer as these are two distinct categories of isomers.





















![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 50 Milligrams](https://m.media-amazon.com/images/I/81HAPnASH5L._AC_UL320_.jpg)

![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 250 Milligrams](https://m.media-amazon.com/images/I/71EL45ccGFS._AC_UL320_.jpg)
![Zeatin, Trans Isomer [6-(4-Hydroxy-3-methylbut-2-enylamino) purine)], 100 Milligrams](https://m.media-amazon.com/images/I/71-dKlpNLrS._AC_UL320_.jpg)


















