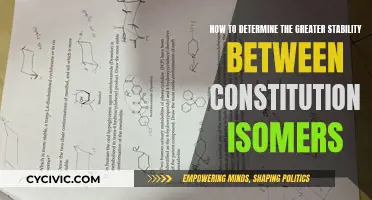
Constitutional isomers and conformational isomers are types of isomerism, a concept in chemistry that deals with molecules that have the same molecular formula but differ in structure. Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of their atoms. On the other hand, conformational isomers, or conformers, have the same molecular formula and connectivity but differ in the rotation around a single sigma bond, resulting in different spatial arrangements. To determine if molecules are constitutional isomers, one must compare the molecular formulas and the connectivity of the atoms. If the formulas are the same but the atoms are connected differently, they are constitutional isomers. For conformational isomers, it is crucial to examine the rotation around a single bond; if the molecules are identical except for a single bond rotation, they are conformational isomers.
| Characteristics | Values |
|---|---|
| Definition of conformational isomers | Molecules that can be superimposed on each other through rotation of bonds or through rotation of the molecule itself |
| Definition of constitutional isomers | Compounds with the same molecular formula but different connectivity of atoms |
| How to identify conformational isomers | Check if the molecules have the same molecular formula and connectivity |
| How to identify constitutional isomers | Check if the molecules have the same molecular formula and different connectivity |
| Other names for conformational isomers | Conformers, geometric isomers |
| Other names for constitutional isomers | Structural isomers |
Explore related products
What You'll Learn
- Constitutional isomers have the same molecular formula but different connectivity of atoms
- Conformational isomers have the same atomic connectivity but different bond rotation
- To identify constitutional isomers, check the number of carbons and heteroatoms
- Constitutional isomers can be distinguished by their different physical and chemical properties
- Constitutional isomers are considered identical copies of the same molecule

Constitutional isomers have the same molecular formula but different connectivity of atoms
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivity of atoms. This means that constitutional isomers have the same types and numbers of atoms but differ in the way these atoms are bonded or connected to each other.
To determine whether two molecules are constitutional isomers, follow these steps:
- Ensure the molecules have the same molecular formula: Check if the two molecules have the same number of atoms of each element. This step is crucial as isomers always have the same chemical formula, and compounds with different chemical formulas are not isomers but completely different compounds.
- Check the connectivity of the atoms: Compare the bonding patterns or structures of the molecules. If the atoms are connected differently, the molecules are constitutional isomers. For example, butane (C4H10) and isobutane (C4H10) have the same molecular formula but different structures. Butane has a straight chain structure, while isobutane has a branched chain structure. This difference in connectivity classifies them as constitutional isomers.
- Assess the degree of unsaturation or the Index of Hydrogen Deficiency (IHD): The IHD is a calculation used to determine the degree of unsaturation in a molecule, indicating the number of rings, double bonds, and triple bonds present. It is calculated using the formula: IHD = (2C + 2 - H + N - X)/2, where C is the number of carbons, H is the number of hydrogens, N is the number of nitrogens, and X is the number of halogens. By comparing the IHD values of the molecules, you can further determine their relationship.
It is important to note that conformers or conformational isomers are different from constitutional isomers. Conformers have the same molecular formula and connectivity but differ by rotation around a single sigma bond without breaking any bonds. These rotations lead to different spatial arrangements of the molecules.
Balancing Act: Constitution's Power Play
You may want to see also

Conformational isomers have the same atomic connectivity but different bond rotation
Conformational isomers are separate variants of the same molecule. They have the same atomic connectivity and bond structure but differ in their spatial arrangement. This difference in spatial arrangement is due to rotation around a single bond.
For example, two molecules may appear different but are, in fact, conformational isomers and thus the same molecule if one has a C-C bond that has been rotated. This rotation changes the spatial arrangement of the atoms, but the underlying connectivity remains the same.
Conformational isomerism is also known as rotamerism, as these isomers can be converted into one another by rotation around a C-C single bond. This rotation is not completely free due to repulsive interactions between the electron clouds of C-H bonds, which results in torsional strain.
Conformational isomers are not considered to be isomers because of their identical bond structure. However, they are distinct from one another and can be interconverted very quickly. This rapid interconversion means that conformational isomers are often considered to be the same compound.
Constitutional isomers, on the other hand, have the same chemical formula but a different connectivity of atoms. This means that constitutional isomers have a different bond structure, and are thus different compounds from one another.
Illinois' Constitutional Evolution: A Historical Overview
You may want to see also

To identify constitutional isomers, check the number of carbons and heteroatoms
Constitutional isomers are compounds with the same molecular formula but different atomic connectivity. They are considered different conformations of the same molecule, resulting from rotation around a single bond. To identify constitutional isomers, one can check the number of carbons and heteroatoms, as well as compare the degree of unsaturation (Hydrogen Deficiency Index). If all the atoms are the same and the molecules have the same HDI, then they are likely constitutional isomers.
For example, ethanol (the drinking alcohol) and dimethyl ether both have the formula C2H6O and the same molecular mass. However, they have different physical and chemical properties due to the different connectivity of their atoms. Thus, they are constitutional isomers.
Another example is butane and cyclobutane, which have different configurations but the same formula. Playing around with different configurations can help identify constitutional isomers.
It is important to remember that molecules are free to move around and can adopt different conformations. Just because they are drawn a certain way does not mean that is the only way of showing their structure. For instance, two molecules might be stereoisomers of each other, but they cannot be stereoisomers and constitutional isomers at the same time.
Constitutional isomers can have the same or different functional groups. For instance, ethanol and propanol are constitutional isomers of each other because they have the same functional group (OH) located at different points on the carbon skeleton. On the other hand, ethanol and dimethyl ether are constitutional isomers because they have the same molecular formula, despite having different functional groups.
While counting the number of carbons and heteroatoms is a good starting point for identifying constitutional isomers, it is not always sufficient. For larger molecules, it may be necessary to name the molecules according to IUPAC nomenclature rules to be absolutely sure.
George Washington's Influence on the US Constitution
You may want to see also
Explore related products

Constitutional isomers can be distinguished by their different physical and chemical properties
Constitutional isomers are compounds with the same molecular formula but different connectivity. In other words, constitutional isomers have the same number of atoms but differ in the way the atoms are connected to one another. For example, ethanol (drinking alcohol) and dimethyl ether both have the formula C2H6O and the same molecular mass, but they have completely different physical and chemical properties due to the different connectivity of their atoms.
It is important to remember that molecules are free to move around and can adopt different conformations. Just because they are drawn in one way does not mean that is the only way of showing an accurate structure for a compound. For example, two molecules may be different conformations of the same molecule due to rotation around a single bond. In such cases, they are considered identical copies of the same molecule rather than isomers.
Additionally, constitutional isomers can have the same or different functional groups. As long as the molecular formula is the same, but the atomic connectivity is different, they are classified as constitutional isomers. For example, two molecules may have the same functional group located at different points on the carbon skeleton.
Exploring the Constitution: Military Force at Home
You may want to see also

Constitutional isomers are considered identical copies of the same molecule
Constitutional isomers are compounds with the same molecular formula but different connectivity of atoms. They are considered identical copies of the same molecule because they have the same chemical formula and the same number of atoms, but the atoms are connected differently. For example, butane (C4H10) and isobutane (C4H10) have the same molecular formula but different structures. Butane has a straight chain, while isobutane has a branched chain. This difference in connectivity classifies them as constitutional isomers.
To identify constitutional isomers, it is important to first ensure that the molecules have the same molecular formula. This is a crucial step because if the chemical formulas are different, then the compounds are completely different, and there is no isomeric relationship. Once it is established that the compounds have the same formula, the next step is to check the connectivity of the atoms. If the atoms are connected differently, then they are constitutional isomers.
The concept of constitutional isomers is particularly relevant in organic chemistry, where there are numerous ways to connect carbon atoms differently and synthesize new molecules. This leads to molecules with similar structures but significantly different properties. For example, ethanol (C2H6O) and dimethyl ether (C2H6O) have the same molecular mass, but their physical and chemical properties differ due to the distinct connectivity of their atoms.
It is worth noting that constitutional isomers are different from conformational isomers. While constitutional isomers have different atomic connectivity, conformational isomers have the same atomic connectivity but differ in bond rotation. Conformational isomers are considered the same molecule in different rotational states, and these rotations occur very rapidly, leading to the interpretation of conformational isomers as the same compound.
American Indians: Constitution Influencers or Silent Bystanders?
You may want to see also
Frequently asked questions
If two molecules have the same molecular formula and connectivity, but differ by rotation around a single sigma bond, they are conformational isomers.
If two molecules have the same molecular formula but different connectivity, they are constitutional isomers.
The easiest way to determine if molecules are constitutional isomers is to count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index). If all the atoms are the same and the molecules have the same HDI, they are constitutional isomers.
Butane (C4H10) and isobutane (C4H10) have the same molecular formula but different structures. Butane has a straight chain, while isobutane has a branched chain. This difference in connectivity classifies them as constitutional isomers.