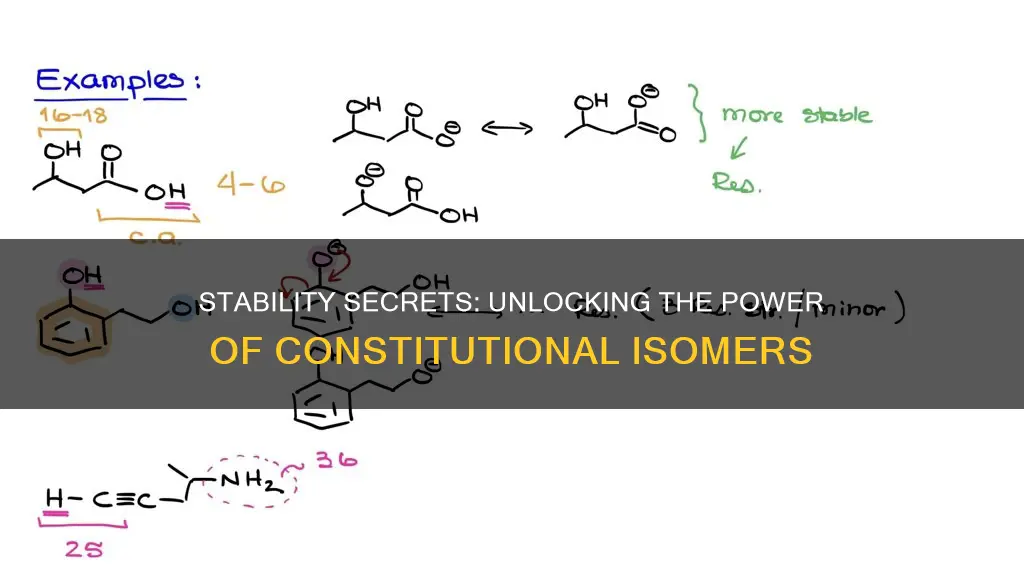
Constitutional isomers are compounds with the same molecular formula but different atomic connectivity. To determine the greater stability between constitutional isomers, one can consider the heat of combustion, where lower values indicate a more stable compound. This is because compounds with lower heat values already reside in a low-energy state. Another method to determine the stability of alkenes is to measure the heats of hydrogenation (ΔH°hydrog) for two double-bond isomers to find their difference and determine their relative stabilities.
Explore related products
What You'll Learn

Compare the number of carbons and the degree of unsaturation
To determine whether two molecules are constitutional isomers, one of the first steps is to compare the number of carbons and the degree of unsaturation. This is done by counting the number of atoms and calculating the Index of Hydrogen Deficiency (IHD) or Hydrogen Deficiency Index (HDI). If the number of atoms is the same and the IHD/HDI is identical, the molecules are likely constitutional isomers.
The IHD is a measure of the number of hydrogen pairs missing from a molecule compared to a fully saturated hydrocarbon. It helps determine the degree of unsaturation, which refers to the number of double bonds, rings, or triple bonds in a molecule. Each pi bond, ring, or triple bond reduces the hydrogen count by two. For example, a triple bond counts as two IHD. Oxygens are ignored, halogens count as one hydrogen, and nitrogens count as negative one.
Constitutional isomers are compounds with the same molecular formula but different connectivity. For example, ethanol (drinking alcohol) and dimethyl ether have the same molecular formula, C2H6O, but different atomic connectivity, making them constitutional isomers. Another example is butane (CH3-CH2-CH2-CH3) and isobutane ((CH3)2CH-CH3), which both have the molecular formula C4H10 but differ in their atomic connectivity.
While comparing the number of carbons and the degree of unsaturation is a crucial first step in identifying constitutional isomers, it may not be sufficient for larger molecules. In such cases, naming the molecules according to IUPAC nomenclature rules may be necessary to ensure accurate identification of constitutional isomers.
Electrons and Charge: Understanding Coulomb's Law
You may want to see also

Assess the connectivity of atoms
Constitutional isomers are compounds with the same molecular formula but different connectivity of atoms. They can have the same or different functional groups. For example, butane and isobutane have the same molecular formula (C4H10) but different connectivities. Butane has an uninterrupted chain of carbon atoms, while isobutane has only three carbon atoms connected in sequence. Another example is ethanol and dimethyl ether, which have the same molecular formula (C2H6O) but different atomic connectivities. The atomic connectivity in ethanol is C—C—O, with the oxygen atom being part of an alcohol. In contrast, the C—O—C connectivity in dimethyl ether forms an ether.
To determine if two molecules are constitutional isomers, you can follow these steps:
- Check if the molecules have the same molecular formula. If they do not, they cannot be constitutional isomers.
- Count the number of carbons and heteroatoms in each molecule.
- Compare the degree of unsaturation or the Hydrogen Deficiency Index (HDI) of the molecules. If all the atoms are the same and the molecules have the same HDI, they are likely constitutional isomers.
- Assess the connectivity of atoms by identifying landmark atoms. If the connections are the same, the compounds are identical; if not, they are constitutional isomers.
It is important to note that stereoisomers are different from constitutional isomers. Stereoisomers have the same connectivity but differ in the arrangement of their atoms in space. For example, two molecules of 2-hexene are stereoisomers because they have the same connectivity but differ in the arrangement of their groups in space about the double bond.
The stability of constitutional isomers can be determined by comparing the heat of combustion. The compound with lower combustion heat is more stable because it exists in a lower energy state. For instance, 2,2-dimethylpropane has a lower heat of combustion than pentane, making it the more stable isomer.
Federal Judges: Appointed or Elected?
You may want to see also

Evaluate the heat of combustion
Constitutional isomers are compounds with the same molecular formula but different structural arrangements. To determine if two molecules are constitutional isomers, you should first check if all non-hydrogen atoms and the Index of Hydrogen Deficiency (IHD) are identical. If they differ, the compounds are not isomers. If they are identical, you should then assess the connectivity of the atoms. If the connections are the same, the compounds are identical; if not, they are constitutional isomers.
Now, to evaluate the heat of combustion, we can use this value as an effective measure to determine the relative stability of isomers. The heat of combustion is the energy released as heat when a compound undergoes complete combustion with oxygen under standard conditions. A lower heat of combustion indicates a more stable compound because it already resides in a low-energy state.
To calculate the heat of combustion, you can use Hess's law, which states that the enthalpies of the products and reactants are the same. Start by writing the balanced equation of combustion of the substance. Then, look up and add the enthalpies of formation for the reactants and products. Finally, subtract the enthalpies of the reactants from the product and convert the units to kilojoules.
The heat of combustion is also useful for analyzing the amount of energy in a given fuel. The higher heating value (HHV) is a measure of the thermal energy produced by the complete combustion of a fuel, and it is determined by bringing all the products of combustion back to the original pre-combustion temperature. The HHV is useful for fuels that contain water prior to burning, such as wood or coal. The lower heating value (LHV) is another measure that accounts for energy losses, such as the energy used to vaporize water.
In summary, the heat of combustion is a key concept in understanding the relative stability of isomers and analyzing the energy content of fuels. By calculating and comparing the heat of combustion for different isomers, we can determine which isomer is more stable.
Founders' Ratification Rift: Constitution's Contentious Journey
You may want to see also
Explore related products
$78.04 $299

Analyse the number of substituents
When comparing constitutional isomers, it is important to first understand what defines these types of isomers. Constitutional isomers are compounds with the same molecular formula but different structural arrangements or connectivity of atoms. This means that the atoms in constitutional isomers are the same, but they are connected differently.
To determine if two molecules are constitutional isomers, one method is to count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index or Index of Hydrogen Deficiency). If all the atoms are the same and the molecules have the same HDI, then they are constitutional isomers. The Hydrogen Deficiency Index is particularly useful in distinguishing between different types of compounds.
However, this method may not always be accurate, especially for large molecules. In such cases, it is recommended to name the molecules according to IUPAC nomenclature rules to be certain.
Now, to address the specific request of analysing the number of substituents to determine the greater stability between constitutional isomers, it is important to clarify that the stability of isomers is typically determined by the heat of combustion. Lower heat of combustion indicates greater stability as it means the compound already resides in a low-energy state.
While there is limited information specifically on how the number of substituents affects the stability of constitutional isomers, it is mentioned that alkyl-substituted polycyclic aromatic compounds (aPAHs) have a significant effect on the toxicity of mixtures compared to their parent PAHs, especially in crude oils. This suggests that the number and type of substituents can influence the properties and behaviour of compounds, which may include stability.
In summary, while the number of substituents may play a role in the overall stability of constitutional isomers, it is not the only factor, and other factors such as the heat of combustion are typically used as a measure of relative stability.
Lawyers and the Framing of the Constitution
You may want to see also

Consider the bond strengths
When considering the bond strengths of constitutional isomers, it is important to remember that these isomers have the same molecular formula but differ in their structural arrangement. This means that the connectivity of atoms varies between the isomers, resulting in distinct chemical and physical properties.
In the context of bond strengths, let's consider alkenes as an example. The stability of alkenes is influenced by the number of substituents. The more substituents an alkene has, the greater its stability. This is because a bond between an sp2 carbon and an sp3 carbon is stronger than a bond between two sp3 carbons. Consequently, an alkene with more substituents will have a higher ratio of sp3-sp2 bonds to sp3-sp3 bonds, contributing to its increased stability.
Now, let's delve into the specifics of constitutional isomers. Butane (C4H10) and isobutane (C4H10) are constitutional isomers with different carbon backbone structures. Butane has an uninterrupted chain of carbon atoms, while isobutane has three carbon atoms in sequence with the fourth carbon atom bonded as a "branch." This variation in the carbon backbone alters the connectivity of the atoms and, consequently, the stability of the molecule.
Additionally, consider the isomers 1-propanol and 2-propanol. Both possess a hydroxyl group, but it is positioned on different carbon atoms. This variation in the location of functional groups within the molecule can influence the stability of the isomers.
Furthermore, when examining bond strengths, it is worth noting that stereoisomers are another type of isomerism that arises from the different arrangements of groups in space around a double bond. While they share the same connectivity, stereoisomers are distinct from constitutional isomers. Stereoisomers can be further classified into configurational stereoisomers and conformational stereoisomers, with the former having a precise spatial arrangement of groups, and the latter requiring rotation about a double bond or dissociation of single bonds to become superimposable.
In conclusion, when considering the bond strengths between constitutional isomers, it is crucial to examine the number of substituents, the arrangement of functional groups, and the variation in carbon backbone structures. These factors influence the stability of the isomers, with stronger bonds contributing to greater stability.
Texas Constitution of 1836: Religious Freedom Impact
You may want to see also
Frequently asked questions
Compounds with the same molecular formula but different atomic connectivity are called constitutional isomers.
First, check if all non-hydrogen atoms and the Index of Hydrogen Deficiency (IHD) are identical. If they differ, the compounds are different. If they match, assess the connectivity by identifying landmark atoms. If the connections are the same, the compounds are identical; if not, they are constitutional isomers.
The heat of combustion helps determine the stability of isomers; less heat released means greater stability. This indicates that the isomer with lower combustion heat exists in a lower energy state, making it more stable.
The heat of combustion of 2,2-dimethylpropane is lower than that of pentane, making it more stable.
The number of substituents contributes to the stability of alkenes. The more substituents there are, the greater the stabilization of the alkene.