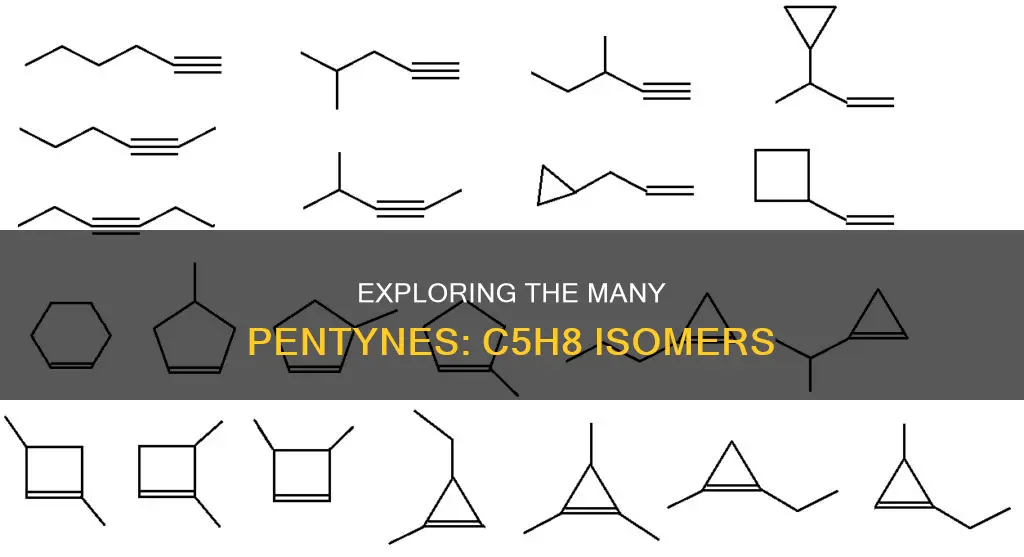
The molecular formula C5H8 refers to several hydrocarbons, including pentynes, pentadienes, butadiene derivatives, cyclopentene, and cyclobutane derivatives. Pentyne isomers of C5H8 include 1-Pentyne, 2-Pentyne, and 3-Methyl-1-butyne or isopentyne. In this context, isomers are molecules with the same molecular formula but different structural formulas, exhibiting isomerism in alkanes, alkenes, and alkynes. The number of possible isomers can be determined using a formula that considers the number of carbon and hydrogen atoms, as well as any halogen or nitrogen atoms present.
| Characteristics | Values |
|---|---|
| Molecular formula | C5H8 |
| Molecular mass | 68 |
| Types of isomers | Structural, carbon chain, cycloalkene, alkyne, stereoisomers, R/S optical isomers, E/Z isomers (cis trans) |
| Number of double bonds or rings | Calculated using the formula: No. of Unsaturations=C-\frac{2}-\frac{2}+\frac{2}+1 |
Explore related products
What You'll Learn
- Pentynes: 1-Pentyne, 2-Pentyne, 3-Methyl-1-butyne
- Pentadienes: 1,2-Pentadiene, 1,3-Pentadiene, 1,4-Pentadiene, 2,3-Pentadiene
- Butadiene derivatives: 3-Methyl-1,2-butadiene, 2-Methyl-1,3-butadiene
- Cyclopentene and Cyclobutane derivatives
- Structural isomers: R/S optical isomers, E/Z isomers, carbon chain isomers

Pentynes: 1-Pentyne, 2-Pentyne, 3-Methyl-1-butyne
There are three pentyne isomers of C5H8: 1-pentyne, 2-pentyne, and 3-methyl-1-butyne (also known as isopentyne). These compounds are hydrocarbons with the molecular formula C5H8, meaning they contain five carbon atoms and eight hydrogen atoms.
Starting with 1-pentyne, it is an alkyne with the triple bond between the first and second carbon atoms. The chemical structure of 1-pentyne is linear, and it is the simplest form of the pentyne isomers.
2-Pentyne, on the other hand, has the triple bond between the second and third carbon atoms. This isomer may have a slightly more complex structure than 1-pentyne due to the potential for different arrangements of the carbon atoms and hydrogen atoms.
3-Methyl-1-butyne, as the name suggests, has a methyl group attached to the third carbon atom of the butyne chain. This isomer has a branched structure due to the presence of the methyl group. It is also known as isopentyne and has a CAS number of 598-23-2. 3-Methyl-1-butyne is a useful reactant in various synthetic processes, including the ruthenium-catalyzed synthesis of functionalized cycloheptadienes and the titanium-catalyzed [2+2+1] multicomponent pyrrole synthesis. It also serves as a precursor in the total syntheses of several compounds, such as (+)-frondosin A, (−)-citrinadin A, (+)-citrinadin B, and coraxeniolide A.
In summary, while all three isomers share the same molecular formula, they differ in the arrangement of their carbon and hydrogen atoms, resulting in unique chemical structures and properties. These isomers of pentyne play essential roles in various chemical processes and synthetic reactions, contributing to their significance in organic chemistry and related fields.
CRC's Constitutional Status in Myanmar: A Legal Perspective
You may want to see also

Pentadienes: 1,2-Pentadiene, 1,3-Pentadiene, 1,4-Pentadiene, 2,3-Pentadiene
The molecular formula C5H8 refers to several hydrocarbons, including pentynes and pentadienes. This response will focus on the four pentadienes isomers: 1,2-Pentadiene, 1,3-Pentadiene, 1,4-Pentadiene, and 2,3-Pentadiene.
1,2-Pentadiene
1,2-Pentadiene, with the molecular formula C5H8, is an unsaturated hydrocarbon with two double bonds between carbon atoms. It has a total of five carbon atoms and eight hydrogen atoms. The double bonds are located between the first and second carbon atoms in the molecule, giving it the name "1,2-Pentadiene."
1,3-Pentadiene
1,3-Pentadiene is another isomer of pentadiene with the molecular formula C5H8. It also has five carbon atoms and eight hydrogen atoms but differs in the location of its double bonds. In 1,3-Pentadiene, there are two cis-z isomers, and the double bonds are located between the first and third carbon atoms, hence the name "1,3-Pentadiene."
1,4-Pentadiene
1,4-Pentadiene, with the molecular formula C5H8, is the third isomer of pentadiene. Like its counterparts, it has five carbon atoms and eight hydrogen atoms. The double bonds in this isomer are located between the first and fourth carbon atoms, leading to its designation as "1,4-Pentadiene."
2,3-Pentadiene
2,3-Pentadiene is the fourth isomer of pentadiene with the molecular formula C5H8. It shares the same carbon and hydrogen atom composition as the other isomers. However, in 2,3-Pentadiene, the double bonds are positioned between the second and third carbon atoms, giving rise to its name. 2,3-Pentadiene exhibits geometric isomerism, with two axial isomers known as (Ra)-2,3-Pentadiene and (Sa)-2,3-Pentadiene.
In summary, the molecular formula C5H8 encompasses various pentadiene isomers, each differing in the placement of carbon-carbon double bonds. These isomers play a significant role in organic chemistry and contribute to the diverse nature of hydrocarbons.
Gene Regulation: Non-Constitutive Genes and Their Control
You may want to see also

Butadiene derivatives: 3-Methyl-1,2-butadiene, 2-Methyl-1,3-butadiene
The molecular formula C5H8 may refer to various hydrocarbons, including butadiene derivatives: 3-Methyl-1,2-butadiene and 2-Methyl-1,3-butadiene (also known as isoprene).
3-Methyl-1,2-butadiene
3-Methyl-1,2-butadiene is a derivative of butadiene with the molecular formula C5H8. It is identified with the CAS number 598-25-4. Unfortunately, I could not find more specific information about this compound.
2-Methyl-1,3-butadiene (Isoprene)
2-Methyl-1,3-butadiene, also known as isoprene, is a common volatile organic compound with the formula CH2=C(CH3)−CH=CH2. In its pure form, it is a colorless volatile liquid. It is produced by many plants and animals, including humans, and its polymers are the main component of natural rubber.
Isoprene was named by C. G. Williams in 1860 after obtaining it from the pyrolysis of natural rubber. Williams correctly deduced the mass shares of carbon and hydrogen but arrived at an incorrect formula, C10H8, as the modern atomic weight of carbon was not yet adopted. Isoprene is made through the methyl-erythritol 4-phosphate pathway (MEP pathway) in the chloroplasts of plants. One of the pathway's end products, dimethylallyl pyrophosphate (DMAPP), is cleaved by the enzyme isoprene synthase to form isoprene and diphosphate.
Isoprene emission increases dramatically with temperature, peaking at around 40°C. This has led to the hypothesis that isoprene may protect plants against heat stress. About 800,000 metric tons of isoprene are produced annually, with 95% used to synthesize cis-1,4-polyisoprene, a synthetic version of natural rubber.
The President's Powers: Understanding US Constitutional Limits
You may want to see also

Cyclopentene and Cyclobutane derivatives
The molecular formula C5H8 refers to several hydrocarbons, including pentynes, pentadienes, butadiene derivatives, cyclopentene, and cyclobutane derivatives.
Cyclopentene Derivatives
Cyclopentene is an organic compound with the formula C5H8. It is a colourless liquid with an unpleasant odour. Cyclopentene derivatives are obtained from cyclopentadiene, which is produced from coal tar and by steam cracking of naphtha. Cyclopentadiene is a highly reactive diene due to its ability to undergo minimal distortion during the Diels-Alder reaction.
Cyclopentadiene can substitute one or more hydrogens, forming derivatives with covalent bonds. For example, the rhodocene derivative is produced from the rhodocene monomer in protic solvents. Cyclopentadiene is also used as a precursor to cyclopentadienyl-based complexes and comonomers. Semi-hydrogenation of cyclopentadiene yields cyclopentene.
Cyclobutane Derivatives
Cyclobutane, with the formula (CH2)4, is a colourless gas that is commercially available in liquefied form. It is a cycloalkane and organic compound synthesised by hydrogenating cyclobutene in the presence of nickel. Cyclobutane itself holds no commercial or biological significance, but its derivatives are important in biology and biotechnology.
Cyclobutane derivatives, also known as cyclobutanes, can be prepared through various methods. For instance, alkenes dimerize upon irradiation with UV light, and 1,4-dihalobutanes can be converted to cyclobutanes through dehalogenation with reducing metals.
Several applications of cyclobutane derivatives are outlined in scientific literature. For example, carboplatin, an anticancer drug, is derived from cyclobutane-1,1-dicarboxylic acid. Additionally, cyclobutanols can be functionalized asymmetrically to form boryl derivatives. Other applications include the synthesis of various chemical compounds and inhibitors.
Stamp Act's Influence: Constitution's Roots
You may want to see also

Structural isomers: R/S optical isomers, E/Z isomers, carbon chain isomers
The molecular formula C5H8 refers to several hydrocarbons, including pentynes and pentadienes. These compounds exhibit isomerism, which is the presence of molecules with the same molecular formula but different structural or spatial arrangements of atoms.
Let's explore the three types of structural isomers mentioned: R/S optical isomers, E/Z isomers, and carbon chain isomers.
R/S Optical Isomers
R/S optical isomers, also known as enantiomers, are stereoisomers that are mirror images of each other. They have the same molecular formula and connectivity of atoms but differ in how the atoms are arranged in space. The "R" and "S" designations are determined by the configuration of atoms around a stereocenter, which is typically a carbon atom bonded to four different substituents. The priority of each substituent is considered, and if traversing from the highest to lowest priority substituent results in a counterclockwise direction, it is designated as "S" ("Sinister" in Latin, meaning left). If the direction is clockwise, it is designated as "R." These optical isomers can have different effects on plane-polarized light, with one rotating it clockwise and the other counterclockwise.
E/Z Isomers
E/Z isomers are geometric isomers that differ in the arrangement of atoms around a carbon-carbon double bond. If the highest priority groups for each carbon are on the same side of the molecule, it is denoted as the "cis" or "Z" isomer. If they are on opposite sides, it is denoted as the "trans" or "E" isomer. The terms "E" and "Z" come from German, with "E" standing for "entgegen," meaning "opposite," and "Z" standing for "zusammen," meaning "together."
Carbon Chain Isomers
Carbon chain isomers are structural isomers that differ in the arrangement of the carbon skeleton of the molecule. They have the same molecular formula but can have either one continuous carbon chain or multiple side groups of carbons branching off. For example, a straight-chain alkane can have a functional group isomer that is a cycloalkane, where the carbons form a ring structure. The number of possible carbon chain isomers increases rapidly with the number of carbon atoms, leading to a high number of structural isomers in larger molecules.
Cato's Letters: Influence on the Constitution
You may want to see also
Frequently asked questions
There are four pentyne constitutional isomers of C5H8: 1-Pentyne, 2-Pentyne, 3-Methyl-1-butyne (or Isopentyne), and 2-Methyl-1-butyne.
Isomers are compounds that have the same molecular formula but differ in structural formula and arrangement of atoms.
The number of isomers can be determined by calculating the number of double bonds or rings in the compound using the formula: No. of Unsaturations=C-(H/2)-(X/2)+(N/2)+1.

























