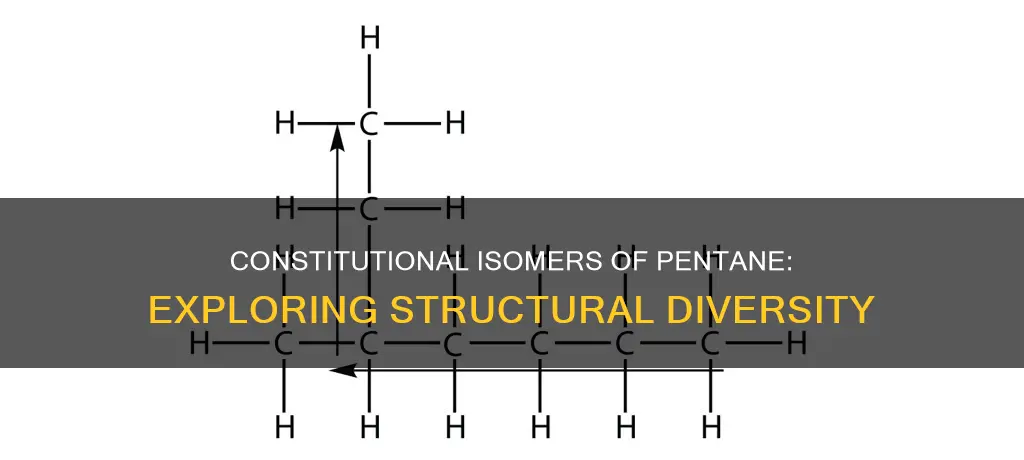
The molecular formula C5H12, commonly known as pentane, has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are distinct structural arrangements of the same molecule, with n-pentane being the straight-chain isomer. While all three isomers share the same molecular formula, they exhibit different properties, including varying boiling points. The existence of multiple compounds with identical chemical formulae but differing structural arrangements and properties is a phenomenon known as isomerism.
| Characteristics | Values |
|---|---|
| Number of constitutional isomers | 3 |
| Molecular formula | C5H12 |
| Names of isomers | n-pentane, isopentane (methyl butane), neopentane (dimethylpropane) |
| CAS numbers | 109-66-0, 78-78-4, 463-82-1 |
| Synonyms | Amyl hydride, 2-Methylbutane, Ethyldimethyl methane, 2,2-Dimethylpropane, tert-Pentane, Tetramethylmethane |
| Boiling points | 36.1°C, 27.7°C, 9.5°C |
Explore related products
What You'll Learn

Pentane has three isomers
Pentane, with the molecular formula C5H12, has three isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are constitutional isomers, which means they have the same chemical formula but differ in the arrangement of atoms and exhibit distinct properties, such as varying boiling points.
The compound on the far left is typically referred to as pentane or n-pentane, and it is characterized by having all five carbon atoms arranged in a continuous, straight chain. This isomer is sometimes referred to as a "normal alkane" or "straight-chain alkane" to emphasize the linear arrangement of its carbon atoms.
The middle compound is isopentane, or methyl butane. Like isobutane, isopentane has a CH3 branch that extends from the second carbon atom of the continuous carbon chain. This branching structure differentiates it from n-pentane and gives rise to unique properties.
The compound on the far right, discovered after the other two, is named neopentane, derived from the Greek word "neos" meaning "new." Neopentane, also known as dimethylpropane, has a distinct structure that sets it apart from the other two isomers.
While these three isomers of pentane share the same molecular formula, C5H12, they exhibit isomerism, a phenomenon where compounds have identical chemical formulae but differ in their atomic arrangement and properties. This variation in atomic arrangement results in distinct conformers, with 13 identical structures and three unique ones, contributing to the overall diversity of pentane's isomers.
The US Constitution: Democracy's Founding Principles
You may want to see also

n-Pentane is the straight-chain isomer
Pentane, with the molecular formula C5H12, has three isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are constitutional isomers, which means they have the same chemical formula but differ in the arrangement of atoms in the molecule.
N-Pentane, also known as normal-pentane or simply pentane, is the straight-chain isomer of the group. It has the chemical formula CH3(CH2)3CH3, with a linear structure of five carbon atoms bonded to each other in a chain, and each carbon atom (except the end ones) is bonded to two hydrogen atoms. This structure can be written as CH3-CH2-CH2-CH2-CH3, highlighting the single covalent bonds between the carbon atoms.
The straight-chain structure of n-pentane gives it distinct properties compared to its isomeric counterparts. For example, n-pentane has a lower boiling point than isopentane and neopentane due to its non-branched structure, which results in weaker intermolecular forces. This also contributes to n-pentane's higher volatility, making it easier to vaporize than its isomers.
The non-branched structure of n-pentane also affects its reactivity and chemical behaviour. The symmetrical structure of the carbon chain can impact the molecule's reactivity patterns, often making it less reactive than its branched isomers. This linear structure also affects the molecule's physical state, as n-pentane is a colourless liquid at room temperature, while isopentane is a volatile liquid, and neopentane is a solid.
Additionally, n-pentane's straight-chain structure contributes to its use as a solvent. The non-polar and linear nature of the molecule allows it to dissolve non-polar substances effectively. This property is utilized in various industrial and laboratory applications, such as a solvent in adhesives, as a blowing agent in foam production, and as a fuel component in some specialty fuels.
US Constitution: Immigration Laws and Their Origins
You may want to see also

Isopentane has a lower boiling point than pentane
Pentane, with the molecular formula C5H12, has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers differ in their physical and chemical properties, particularly in terms of their boiling points.
Isopentane, also known as methylbutane, has a lower boiling point compared to its isomer, n-pentane. This difference in boiling points between isopentane and n-pentane can be attributed to their structural variations. The shape and surface area of these isomeric molecules play a crucial role in determining their respective boiling points.
N-pentane, with a higher surface area of 118 Å^2, exhibits stronger intermolecular forces compared to isopentane, which has a smaller surface area of 112 Å^2. The greater surface area of n-pentane allows for more attractive interactions with other molecules, resulting in a higher boiling point of approximately 36.1°C. On the other hand, isopentane, with its lower surface area, experiences weaker intermolecular forces, leading to a lower boiling point.
The shape of the molecules also influences their boiling points. Neopentane, another isomer of pentane, has a spheroid shape that facilitates its packing into a solid structure. This efficient packing contributes to its lower boiling point of 10°C. While the specific boiling point of isopentane is not readily available, it is understood to be lower than that of n-pentane due to its structural characteristics.
In summary, the lower boiling point of isopentane compared to n-pentane can be attributed to its smaller surface area and shape, which result in weaker intermolecular forces and a lower boiling temperature. This relationship between structure and physical properties is a fascinating aspect of isomerism, where compounds with the same chemical formula exhibit distinct characteristics due to differences in the arrangement of atoms.
Australian Constitution: Amendments and Their Impact
You may want to see also
Explore related products

Neopentane was discovered after the other two isomers
Pentane, with the molecular formula C5H12, has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are distinct from each other and have different chemical structures, despite sharing the same molecular formula.
Neopentane, also known as 2,2-dimethylpropane or tert-pentane, was indeed discovered after the other two isomers of pentane. It is the third isomer, with the chemical structure C(CH3)4. Neopentane has a lower boiling point compared to n-pentane and isopentane, with values of 9.5°C, 36.1°C, and 27.7°C, respectively. This difference in boiling points is one of the properties that set neopentane apart from the other two isomers.
The discovery of neopentane came about through the exploration of organic molecules and their ability to exist as different constitutional isomers. While there are 16 potential conformers of pentane mixtures, only three are unique due to the presence of 13 identical structures. This led to the identification of the three distinct isomers of pentane, with neopentane being the last one discovered.
The naming of neopentane reflects its place in the timeline of discovery. The prefix "neo-" in its name is derived from the Greek word "neos," meaning "new." This prefix is often used in chemistry to indicate a newer version or discovery, which aligns with neopentane being the last isomer of pentane to be found.
In summary, neopentane, or tert-pentane, was indeed discovered after the other two isomers of pentane. Its name reflects its late arrival, and it exhibits distinct properties, such as a lower boiling point, that differentiate it from n-pentane and isopentane. The exploration of organic molecules and their isomeric forms led to the eventual discovery of neopentane as the third and final isomer of pentane.
The Constitution: Correcting Flaws and Building a Nation
You may want to see also

The likelihood of a mixture of pentane conformers can be calculated
Pentane, with the molecular formula C5H12, has three structural isomers: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane). These isomers are distinct from each other and have different chemical structures despite sharing the same chemical formula.
The likelihood of obtaining a mixture of pentane conformers can be calculated by considering the different constitutional isomers that pentane can exhibit. There are 16 potential conformers of pentane, but many of these structures are identical, resulting in only three unique conformers.
To calculate the probability of specific conformers being present in a mixture, we can use the product rule. This rule allows us to account for identical structures and focus on the distinct conformers. By applying this rule, we can determine the likelihood of finding specific conformers, including identical structures, within the three unique conformers of pentane.
The calculation of conformer probabilities is based on the reagent used and the orientation of collisions within a chemical reaction. These factors influence the likelihood of obtaining a particular mixture of pentane conformers. By considering these variables and utilizing the product rule, we can gain insights into the probabilities associated with the presence of specific conformers in a mixture, ultimately helping us understand and predict the behavior of pentane molecules in different reactions.
In summary, the likelihood of obtaining a mixture of pentane conformers can be calculated by considering the three unique conformers of pentane and applying the product rule to account for identical structures. This calculation takes into account the reagent and collision orientation, providing valuable information about the probabilities of specific conformers in a given mixture.
Switzerland's Constitution: A Comprehensive Chapter Guide
You may want to see also
Frequently asked questions
There are three constitutional isomers of pentane: n-pentane, isopentane (methyl butane), and neopentane (dimethylpropane).
The molecular formula of pentane is C5H12.
Isomerism is the phenomenon in which multiple compounds have the same chemical formula but different chemical structures.
The boiling points of the isomers differ. Pentane has a boiling point of 36.1°C, isopentane 27.7°C, and neopentane 9.5°C.