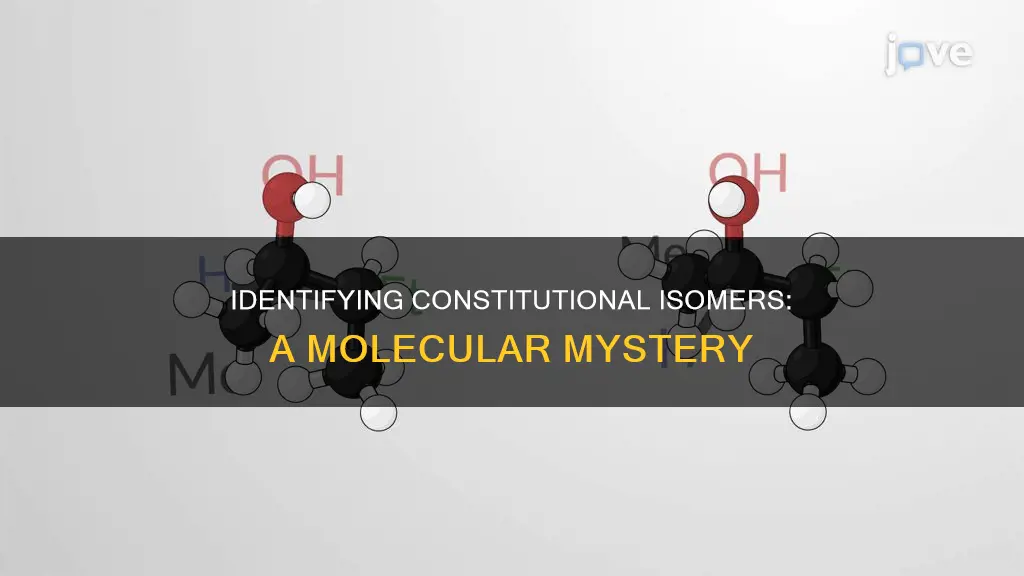
Constitutional isomers are molecules that share the same molecular formula but differ in the connectivity of atoms within the molecules. For instance, ethanol (ethyl alcohol) and dimethyl ether both have the molecular formula C2H6O, but their atomic connections differ, making them constitutional isomers. The HDI (which can be calculated from the molecular formula) helps determine the structural motifs of constitutional isomers, with a higher HDI indicating more possibilities for structural motifs. This understanding of constitutional isomers is crucial in organic chemistry, and being able to identify them is an important skill.
| Characteristics | Values |
|---|---|
| Definition | Molecules with the same molecular formula but a different connectivity of atoms |
| Example | Ethanol (ethyl alcohol) and dimethyl ether |
| HDI | The combination of cycles and double or triple bonds in a molecule gives its HDI value |
| HDI Calculation | Can be calculated from the molecular formula |
Explore related products
What You'll Learn

Constitutional isomers have the same molecular formula
Constitutional isomers are molecules that share the same molecular formula but differ in the connectivity of atoms within the molecules. For instance, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers with the molecular formula C2H6O. Despite possessing identical atoms in identical ratios, the arrangement of these atoms, or the constitution of the molecule, differs. This distinction in structure results in unique properties for each isomer.
The concept of constitutional isomers can be further elucidated through the calculation of the HDI (High Degree of Implication) index. The combination of cycles, double bonds, and triple bonds in a molecule determines its HDI value. Notably, constitutional isomers exhibit identical HDI indexes due to their identical molecular formulas. This knowledge facilitates the drawing of various constitutional isomers with accurate structural motifs.
To determine the potential constitutional isomers of a molecule, one must initially consider its molecular formula. For example, a molecule with the formula C3H6O and an HDI of 1 indicates the presence of either a double bond or a cycle, but not both. Subsequently, one can propose linear structures for the constitutional isomers by focusing on the core structure and subsequently adding the requisite number of hydrogen atoms.
Following the exploration of linear molecules, the introduction of branches or side chains extending from the core atoms can be considered. Additionally, the possibility of cyclic structures, where the molecule forms a closed ring, should be explored. While the number of potential constitutional isomers increases exponentially with the number of atoms, it is unlikely that an instructor would present a problem with more than a dozen structural possibilities. Nonetheless, molecules with 5 to 7 constitutional isomers are commonly encountered in educational contexts.
Compromise: Constitution Ratification's Key
You may want to see also

They have different connectivity of atoms
Constitutional isomers are molecules that have the same molecular formula but differ in the way their atoms are connected. This is also known as structural isomerism. The different connectivity of atoms in constitutional isomers results in distinct physical and chemical properties, despite the molecules having the same composition. For example, ethanol (ethyl alcohol) and dimethyl ether both have the molecular formula C2H6O, but they are different molecules because of the way their atoms are connected. Another example is the pair of isomers, butane and isobutane, which both have the molecular formula C4H10. Butane has an uninterrupted chain of carbon atoms, whereas isobutane has only three carbon atoms connected in sequence, with the fourth carbon atom bonded to the chain as a "branch".
The concept of constitutional isomers is particularly relevant in organic chemistry, where there are no limits to connecting carbon atoms differently and creating new molecules. As the number of carbon atoms increases, the number of possible isomers also increases. For instance, dodecane (C12H26) has 355 possible isomers.
To determine if two molecules are constitutional isomers, one can count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index or HDI). If all the atoms are the same and the molecules have the same HDI, they may be constitutional isomers. However, it is important to note that stereoisomers have the same connectivity among their atoms but differ in their arrangement in three-dimensional space. Therefore, stereoisomers cannot be constitutional isomers.
In summary, constitutional isomers have the same molecular formula but differ in the connectivity of their atoms, resulting in distinct properties. This concept is fundamental in organic chemistry, where the arrangement of atoms within a molecule plays a crucial role in determining its characteristics.
The Constitution and Religion: A Fine Line
You may want to see also

They have different chemical properties
Constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but differ in the way their atoms are connected. For instance, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers as they have the same atoms in identical ratios, but the connections between those atoms differ. This distinction in atomic connectivity results in unique chemical properties for each isomer.
The arrangement of atoms within a molecule is of utmost importance in chemistry as it dictates the molecule's behaviour and reactivity. Even a slight alteration in the atomic structure can lead to significant changes in a molecule's physical and chemical characteristics. This principle is exemplified by constitutional isomers, which, despite sharing a common molecular formula, exhibit distinct chemical properties due to their unique atomic arrangements.
The concept of constitutional isomers underscores the intricate relationship between molecular structure and function. The mere rearrangement of atoms within a molecule can lead to the emergence of novel chemical properties. This highlights the delicate balance within chemical compounds, where subtle changes can have profound effects on their behaviour.
The unique chemical properties of constitutional isomers can be attributed to the altered spatial configuration of their atoms. This deviation in atomic arrangement influences the molecule's reactivity, solubility, melting point, and other physicochemical attributes. Consequently, constitutional isomers with identical molecular formulas can exhibit vastly different behaviours in chemical reactions and biological systems.
The understanding and identification of constitutional isomers are crucial in various scientific disciplines, especially in fields like pharmacology and biochemistry. The distinct chemical properties of these isomers can significantly impact drug design, chemical synthesis, and the interpretation of biochemical reactions. Hence, the study of constitutional isomers contributes to our broader comprehension of molecular behaviour and reactivity.
Money and Felony in North Carolina: How Much Is Too Much?
You may want to see also
Explore related products

They can be identified by counting the number of carbons
To identify constitutional isomers, one must consider the number of carbon atoms and heteroatoms in a molecule, as well as the degree of unsaturation. Constitutional isomers share the same molecular formula but differ in the way their atoms are bonded or connected. For example, butane (C4H10) can have different structures where the four carbons and ten hydrogens are connected differently, making them constitutional isomers. Similarly, C2H6O is the formula for both ethanol and dimethyl ether, which have distinct physical and chemical properties due to the different arrangements of their atoms.
In some cases, constitutional isomers can also have different functional groups. For instance, in the case of C3H8O, two molecules can be constitutional isomers if they have the same functional group (OH) located at different points on the carbon skeleton. On the other hand, one molecule can be an ether with a different functional group, but because it has the same molecular formula, it is still considered a constitutional isomer of the alcohols.
The number of potential constitutional isomers can be narrowed down by considering certain characteristics. For instance, for a compound DBE = 1, the isomers must contain either a double bond or a ring. However, this does not provide an exact count of the number of isomers.
As an example, consider the task of identifying constitutional isomers for molecules with the molecular formula C4H8Cl2. To solve this, one would need to draw bond-line structures to visualize the different arrangements of atoms that satisfy the given molecular formula.
Deadlock Trials: Understanding Count Complexity
You may want to see also

They can be categorised as identical, isomers or different compounds
Constitutional isomers are molecules that share an identical molecular formula but differ in the way their atoms are connected. For instance, ethanol (ethyl alcohol) and dimethyl ether both have the molecular formula C2H6O, but their atomic bonds differ, making them distinct molecules with unique properties. This distinction is crucial, and being able to identify constitutional isomers is an important skill in chemistry.
Constitutional isomers can be categorised in three ways: as identical molecules, as isomers, or as different compounds. If two molecules have the same molecular formula and atomic connections, they are considered identical. However, if the atomic connections differ, they are classified as isomers. Additionally, if two molecules have different molecular formulas and atomic connections, they are regarded as different compounds.
The HDI (High Duplicate Index) value is a useful concept in understanding constitutional isomers. It is calculated from the molecular formula and helps predict the structural motifs of isomers. A higher HDI value indicates more possibilities for structural motifs, but it does not provide an instant answer, especially with complex molecules. To determine possible constitutional isomers, one should start with the molecular formula, derive the HDI, and then explore linear structures before considering side branches and cyclic structures.
The process of categorisation involves examining the molecular formulas and atomic connections. If the formulas match but the atomic connections differ, the molecules are isomers. For example, two molecules with the formula C3H6O may differ in having either a double bond or a cyclic structure, making them isomers. However, if the molecular formulas differ, they are considered different compounds.
In summary, the distinction between identical molecules, isomers, and different compounds rests on comparing molecular formulas and atomic connections. This classification is fundamental in understanding the diverse properties of substances and predicting their structural variations.
Founders' Liberty: Freedom and the Constitution
You may want to see also
Frequently asked questions
Constitutional isomers are molecules that share an identical molecular formula but differ in the way their atoms are connected. For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers of the molecular formula C2H6O.
As constitutional isomers have the same molecular formula, they will also share the same HDI (Hydrogen-Deuterium Exchange) index. This relationship is useful because knowing the HDI index can help in drawing various constitutional isomers with the correct structural motifs.
A straightforward approach to generating constitutional isomers involves first considering linear structures, then exploring possible branches from core atoms, and finally investigating any cyclic structures that can be formed.
A higher HDI index indicates more possibilities for the structural motifs in a molecule. By calculating the HDI index from the molecular formula, you can gain insights into the types of structural features to expect in the molecule, such as the presence of double bonds or cycles.
It is important to recognize that constitutional isomers are distinct molecules with unique properties due to their differing atomic connections. Additionally, the number of possible constitutional isomers increases exponentially with the number of atoms in a molecule, so complexity can rise quickly.











































