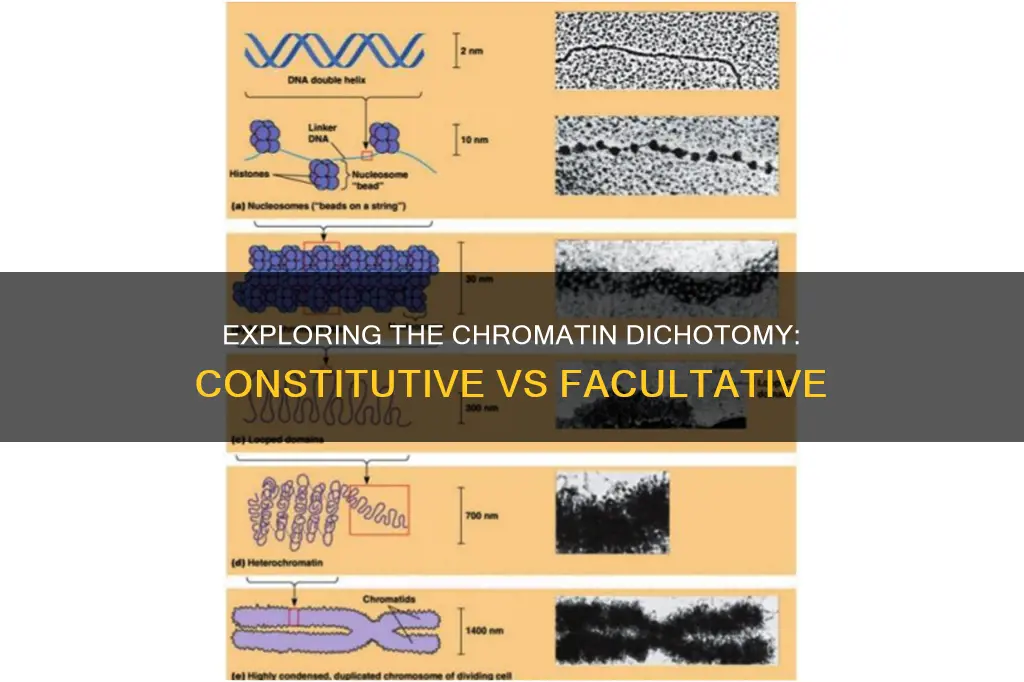
Heterochromatin is a tightly packed form of DNA that comes in two varieties: constitutive heterochromatin and facultative heterochromatin. Both play a role in gene expression. Constitutive heterochromatin is found in the same genomic regions in every cell type and is composed of highly condensed and modified DNA that prevents transcription. Facultative heterochromatin, on the other hand, can form at various chromosomal regions and is less condensed, less stable, and less polymorphic. It is the result of genes that are silenced through mechanisms such as histone deacetylation or Piwi-interacting RNA (piRNA) and can become transcriptionally active under specific developmental or environmental cues.
| Characteristics | Values |
|---|---|
| Definition | Constitutive heterochromatin: Refers to large segments of the genome, occurring at the same genomic regions in every cell type and believed to be devoid of genes. Facultative heterochromatin: Designates genomic regions that have the opportunity to adopt open or compact conformations within temporal and spatial contexts. |
| Formation | Constitutive heterochromatin: Mainly formed at gene-poor regions of pericentromeres and telomeres, consisting of repetitive tandem satellite repeats. Facultative heterochromatin: May form at various chromosomal regions, not characterized by repetitive sequences, and sharing a compact structure with constitutive heterochromatin. |
| Function | Constitutive heterochromatin: Ensures a condensed and transcriptionally inert conformation, protecting the underlying DNA from being accessed for transcription or other transactions. Facultative heterochromatin: Regulates gene expression, with genes that can be silenced or become transcriptionally active in response to developmental or environmental cues. |
| Staining | Constitutive heterochromatin: Stains darker when using the C-banding technique. Facultative heterochromatin: Does not stain with the C-banding technique. |
| Methylation | Constitutive heterochromatin: Marked by histone H3 methylation (H3K9me3) and the presence of HP1 proteins. Facultative heterochromatin: Enriched with H3K27me3 and may exhibit different methylation patterns depending on the organism. |
| Stability | Constitutive heterochromatin: Viewed as a more static structure. Facultative heterochromatin: Less stable and more dynamic. |
Explore related products
$9.92 $18.95
What You'll Learn
- Facultative heterochromatin is less condensed, less stable, and less polymorphic than constitutive heterochromatin
- Facultative heterochromatin can be inherited or arise spontaneously in response to developmental or environmental cues
- Constitutive heterochromatin is found at the same genomic regions in every cell type, while facultative heterochromatin is not
- Facultative heterochromatin is associated with the methylation of H3K27
- Constitutive heterochromatin is necessary for the proper segregation of sister chromatids and centromere function during mitosis

Facultative heterochromatin is less condensed, less stable, and less polymorphic than constitutive heterochromatin
Facultative heterochromatin is a cytological manifestation of epigenetic mechanisms that regulate gene expression. It is not characterized by repetitive sequences, so at the DNA sequence level, it differs from constitutive heterochromatin. However, it has many of the same molecular signatures as constitutive heterochromatin at the nucleosome level. For example, histone hypoacetylation and H3-K9 methylation occur during the formation of the inactive X chromosome (Xi) in female mammals.
The dynamic nature of facultative heterochromatin was first recognized through extensive studies of transposable elements in plants. It was initially described as developmentally regulated heterochromatinization of only one allele of a homologous chromosome pair. Facultative heterochromatin designates genomic regions in the nucleus of a eukaryotic cell that have the opportunity to adopt open or compact conformations within temporal and spatial contexts.
Facultative heterochromatin is also less stable than constitutive heterochromatin. It is not marked by distinctive histone H3 methylation and the presence of HP1 proteins, which are characteristic of constitutive heterochromatin. Instead, it may be enriched in variant histones and non-histone proteins, such as the mammalian inactive X chromosome, which contains variant histones macroH2A.1 and 2.
Media Freedom in African Constitutions: A Comparative Study
You may want to see also

Facultative heterochromatin can be inherited or arise spontaneously in response to developmental or environmental cues
Facultative heterochromatin is a cytological manifestation of epigenetic mechanisms that regulate gene expression. It is not characterised by repetitive sequences, making it entirely different from constitutive heterochromatin at the DNA sequence level. It is the result of genes that are silenced through mechanisms such as histone deacetylation or Piwi-interacting RNA (piRNA) through RNAi. It shares the compact structure of constitutive heterochromatin. However, under specific developmental or environmental cues, it can lose its condensed structure and become transcriptionally active.
The dynamic nature of facultative heterochromatin is further emphasised by its ability to respond to developmental or environmental cues. Under specific conditions, it can lose its condensed structure and become transcriptionally active. This suggests that facultative heterochromatin is more responsive to cellular needs than constitutive heterochromatin. The formation of facultative heterochromatin is also associated with specific developmental processes, such as the regulation of Hox gene expression during the development of Drosophila.
The inheritance of facultative heterochromatin is an intriguing aspect of its biology. When a cell divides, the resulting daughter cells typically contain heterochromatin within the same regions of DNA, leading to epigenetic inheritance. This inheritance can have significant effects on gene expression and cellular function. For example, genetic disorders resulting from mutations in constitutive heterochromatin, such as Roberts syndrome and ICF syndrome, are inherited in an autosomal recessive pattern.
The spontaneous formation of facultative heterochromatin in response to developmental or environmental cues highlights its adaptability. It allows organisms to respond to changing conditions and regulate gene expression accordingly. This spontaneity is a key feature that distinguishes facultative heterochromatin from constitutive heterochromatin, which is more static and consistent across cell types. The ability of facultative heterochromatin to arise spontaneously provides a level of flexibility in gene regulation that is essential for an organism's survival and adaptation to its environment.
Grazing Diet: Small Meals, Big Impact
You may want to see also

Constitutive heterochromatin is found at the same genomic regions in every cell type, while facultative heterochromatin is not
Heterochromatin is a tightly packed form of DNA that comes in two varieties: constitutive heterochromatin and facultative heterochromatin. Both play a role in gene expression. Constitutive heterochromatin is found at the same genomic regions in every cell type, while facultative heterochromatin is not.
Constitutive heterochromatin is formed at the gene-poor regions of pericentromeres and telomeres. It is composed mainly of high-copy-number tandem repeats known as satellite repeats, minisatellite and microsatellite repeats, and transposon repeats. The repeat sequences found at the pericentromeres are not conserved across many species and are regulated by epigenetic modifications. In most organisms, constitutive heterochromatin occurs around the chromosome centromere and near telomeres. It is believed to ensure a condensed and transcriptionally inert chromatin conformation. In humans, constitutive heterochromatin is found on chromosomes 1, 9, 16, and the Y-chromosome. It is usually repetitive and forms structural functions such as centromeres or telomeres, in addition to acting as an attractor for other gene-expression or repression signals.
Facultative heterochromatin, on the other hand, may form at various chromosomal regions, and the regions of DNA packaged in it are not consistent between cell types within a species. It is the result of genes that are silenced through mechanisms such as histone deacetylation or Piwi-interacting RNA (piRNA) through RNAi. It is not repetitive and shares the compact structure of constitutive heterochromatin. However, under specific developmental or environmental cues, it can lose its condensed structure and become transcriptionally active. It is often associated with morphogenesis or differentiation.
The distinction between the two types of heterochromatin lies in their genomic consistency across cell types. While constitutive heterochromatin is found at the same genomic regions in every cell type, facultative heterochromatin is not and can vary between cell types within a species. This variability allows facultative heterochromatin to confer phenotypic differences that are encoded at the chromatin level and can be inherited or arise in response to developmental or environmental cues.
The Constitution: A Slave's Perspective
You may want to see also
Explore related products

Facultative heterochromatin is associated with the methylation of H3K27
Facultative heterochromatin (fHC) is a form of chromatin that can adopt open or compact conformations within temporal and spatial contexts. It is the result of genes that are silenced through mechanisms such as histone deacetylation or Piwi-interacting RNA (piRNA) through RNAi. It is not repetitive and shares a compact structure with constitutive heterochromatin. However, under specific developmental or environmental cues, it can become transcriptionally active.
Methylated lysine 27 on histone H3 (H3K27me) marks repressed facultative heterochromatin, which includes developmentally regulated genes in plants and animals. The mechanisms responsible for the localization of H3K27me are not yet fully understood due to the complexity of epigenetic regulatory networks. Studies have shown that the loss of DNA methylation can trigger H3K27me3 redistribution. In higher eukaryotes, reductions in H3K9me3 and DNA methylation in constitutive heterochromatin have been reported to cause redistribution of H3K27me3.
In a study using Neurospora crassa, a model organism bearing both facultative and constitutive heterochromatin, it was found that the elimination of any member of the DCDC H3K9 methylation complex caused significant changes in the distribution of H3K27me. Regions of facultative heterochromatin lost H3K27me3, while regions typically marked by H3K9me3 became methylated at H3K27. This indicates that H3K27 methylation plays a crucial role in the dynamics of facultative heterochromatin.
Additionally, the loss of HP1, which "reads" H3K9me3, also resulted in substantial changes in the distribution of H3K27me. This further highlights the importance of H3K27 methylation in the regulation of facultative heterochromatin. Overall, these findings contribute to our understanding of the interactions between facultative and constitutive heterochromatin in eukaryotes.
In summary, facultative heterochromatin is associated with the methylation of H3K27, and this methylation plays a significant role in the dynamics and regulation of facultative heterochromatin, particularly in response to developmental and environmental cues.
The Constitution: Guarding Against Tyranny
You may want to see also

Constitutive heterochromatin is necessary for the proper segregation of sister chromatids and centromere function during mitosis
Heterochromatin is a densely packed form of DNA that comes in two varieties: constitutive heterochromatin and facultative heterochromatin. Constitutive heterochromatin is formed at the gene-poor regions of pericentromeres and is crucial for accurate chromosome segregation during mitosis. The pericentromeres consist of repetitive tandem satellite repeats, which vary in size and sequence between different organisms, suggesting that pericentromeric functions are controlled epigenetically.
During mitosis, sister chromatids must be accurately separated and distributed to the two daughter cells. Constitutive heterochromatin, with its highly condensed and transcriptionally inert structure, plays a key role in ensuring proper segregation of sister chromatids. The dense packing of DNA in constitutive heterochromatin prevents it from being accessed by transcription machinery, protecting the underlying DNA during this process.
The highly condensed structure of constitutive heterochromatin is maintained through specific histone modifications, such as histone hypoacetylation, histone H3-Lys9 methylation (H3K9me3), and cytosine methylation. These modifications contribute to the inert state of the chromatin, making it crucial for the proper function of the centromere during mitosis. The centromere is essential for the attachment of spindle fibres, which facilitate the separation and movement of chromatids during cell division.
The presence of constitutive heterochromatin at the pericentromeric regions ensures the stability and integrity of the centromere, allowing for accurate chromosome segregation. The epigenetic regulation of pericentromeric functions adds another layer of control, ensuring that the process is tightly regulated and coordinated. While the specific repeat sequences may vary across species, the overall function of constitutive heterochromatin in chromosome segregation remains conserved.
In summary, constitutive heterochromatin is necessary for the proper segregation of sister chromatids and centromere function during mitosis due to its highly condensed and transcriptionally inert nature. Its presence at the pericentromeric regions provides stability and protection to the underlying DNA, ensuring accurate chromosome separation during cell division. The epigenetic control of pericentromeric functions further contributes to the precise regulation of this vital process.
Founding Fathers: Constitution's Core Reasons
You may want to see also
Frequently asked questions
Constitutive heterochromatin is a condensed and transcriptionally inert conformation of DNA. It is found at the same genomic regions in every cell type and usually does not contain genes. It is formed at the gene-poor regions of pericentromeres and telomeres.
Facultative heterochromatin is a type of heterochromatin that may form at various chromosomal regions. It usually contains genes that must be kept silent upon developmental or environmental cues. It is less condensed, less stable, and less polymorphic than constitutive heterochromatin.
Facultative heterochromatin is a manifestation of epigenetic mechanisms that regulate gene expression. It confers phenotypic differences that can be inherited or spontaneously arise in response to developmental or environmental cues. Constitutive heterochromatin, on the other hand, is often viewed as a more static structure.
Constitutive heterochromatin is marked by distinctive histone H3 methylation and the presence of HP1 proteins. Facultative heterochromatin, however, has less clear chromatin modifications. While both types share some molecular signatures at the nucleosome level, facultative heterochromatin is not characterized by repetitive sequences and is thus entirely different from constitutive heterochromatin at the DNA sequence level.