Isomers are compounds with the same molecular formula but different structures or arrangements. Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of atoms within the molecule. This means the atoms are connected in different ways, leading to different structural formulas. Conformational isomers, on the other hand, have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. These are different forms of the same molecule that can interconvert by rotation around single bonds.
| Characteristics | Conformational Isomers | Constitutional Isomers |
|---|---|---|
| Molecular Formula | Same | Different |
| Connectivity | Same | Different |
| Atoms | Same | Same |
| Bonds | Different | Different |
| Rotations about Single Bonds | Different | Different |
| Interconvertibility | Can be interconverted by rotation around single bonds | N/A |
| Examples | Staggered and eclipsed forms of butane | Isobutane |
Explore related products
What You'll Learn

Constitutional isomers have the same molecular formula
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the way atoms are connected within the molecule. This means that the atoms are bonded differently, leading to different structural formulas. For example, butane (a four-carbon chain) and 2-methylpropane (a one-carbon branch from a three-carbon chain) are constitutional isomers of the chemical formula C4H10. The key difference between constitutional isomers lies in their connectivity or bonding arrangement, rather than the spatial orientation of atoms.
In contrast, conformational isomers have the same molecular formula and connectivity but differ in their spatial orientation due to rotation around a single bond. These isomers are different forms of the same molecule that can interconvert through rotation. For instance, staggered and eclipsed forms of butane are examples of conformational isomers.
It is important to distinguish between structural differences and spatial differences when comparing compounds. While constitutional isomers differ in their bonding patterns, conformational isomers exhibit variations in the rotation about single bonds, resulting in distinct molecular conformations.
Constitutional isomers are a type of stereoisomer, which includes other subtypes such as enantiomers, diastereomers, geometric isomers, and conformers. Stereoisomers share identical molecular formulas and arrangements of atoms but differ in the spatial orientation of groups within the molecule.
In summary, constitutional isomers are characterised by the same molecular formula and different bonding arrangements, while conformational isomers exhibit the same molecular formula, connectivity, and distinct spatial orientations due to rotations around single bonds.
Criminal Acts: What Constitutes a Crime?
You may want to see also

Conformational isomers have the same molecular formula and connectivity
For example, butane (a four-carbon chain) and 2-methylpropane (a one-carbon branch from a three-carbon chain) are structural isomers because they both have the chemical formula C4H10. However, their connectivity is different. The rotation of a single bond can create dynamic molecules with different conformations, or shapes. These different conformations are known as conformational isomers.
It is important to differentiate between constitutional isomers and conformational isomers. Constitutional isomers have the same molecular formula but differ in the connectivity of atoms within the molecule, resulting in different structural formulas. On the other hand, conformational isomers have the same molecular formula and connectivity but differ in the spatial orientation of atoms.
Conformational isomers are a type of stereoisomer, which means they have the same molecular formula and arrangement of atoms but differ in the spatial orientation of groups in the molecule. Stereoisomers include enantiomers, diastereomers, geometric isomers, and conformers. Enantiomers are stereoisomers that are non-superimposable mirror images, while diastereomers are stereoisomers that are not mirror images. Geometric isomers are stereoisomers that differ in the rigidity of double bonds, resulting in different spatial orientations.
In summary, conformational isomers have the same molecular formula and connectivity as their parent molecule but differ in the spatial orientation of atoms due to rotation around a single bond. They are a type of stereoisomer, which is a broader category that includes other subtypes such as enantiomers and diastereomers.
Electoral College: A Constitutional Conundrum?
You may want to see also

Constitutional isomers have different connectivity
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in their connectivity or bonding arrangement. This means that the atoms within the molecule are connected in different ways, resulting in distinct structural formulas. For instance, butane (a four-carbon chain) and 2-methylpropane (a one-carbon branch from a three-carbon chain) are constitutional isomers of the chemical formula C4H10. Their atoms are bonded differently, leading to unique structures.
The distinction between constitutional isomers lies in the connectivity of their atoms. While constitutional isomers share the same molecular formula, the connections between their atoms differ. This variation in bonding patterns results in distinct structural formulas. For example, cyclohexane and methylcyclopentane are constitutional isomers as they possess the same chemical formula, but their atoms are bonded differently.
The concept of constitutional isomers is closely related to the idea of stereoisomers. Stereoisomers have identical molecular formulas and arrangements of atoms, but they differ in the spatial orientation of groups within the molecule. This distinction between constitutional and stereoisomers is crucial in understanding the broader concept of isomers.
Constitutional isomers are a specific type of isomer, characterized by their distinct connectivity of atoms. While they share the same molecular formula, their bonding arrangements differ, resulting in unique structural formulas. This differentiates them from other types of isomers, such as stereoisomers, which have the same molecular formula and atom arrangements but vary in spatial orientation.
The understanding of constitutional isomers is essential in organic chemistry, as it highlights the diverse structural arrangements that molecules can exhibit while maintaining the same molecular formula. By recognizing the differences in connectivity, scientists can classify and study these isomers, contributing to our understanding of chemical compounds and their properties.
Qualities of a Citizen: Constitutional Standards
You may want to see also
Explore related products

Conformational isomers differ in the spatial orientation of atoms
Conformational isomers are essentially the same molecule but differ in the rotation about a single bond. They have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. These are different forms of the same molecule that can interconvert by rotation around single bonds.
For example, butane can have constitutional isomers like isobutane, while its conformational isomers include staggered and eclipsed forms. The rotation about single bonds creates dynamic molecules. When drawing and discussing molecules, it is important to be aware that our drawings are static while the molecules themselves are rotating. Although there are seven sigma bonds in the ethane molecule, rotation about the six carbon-hydrogen bonds does not result in any change in the shape of the molecule because the hydrogen atoms are essentially spherical. Rotation about the carbon-carbon bond, however, results in many different possible molecular conformations.
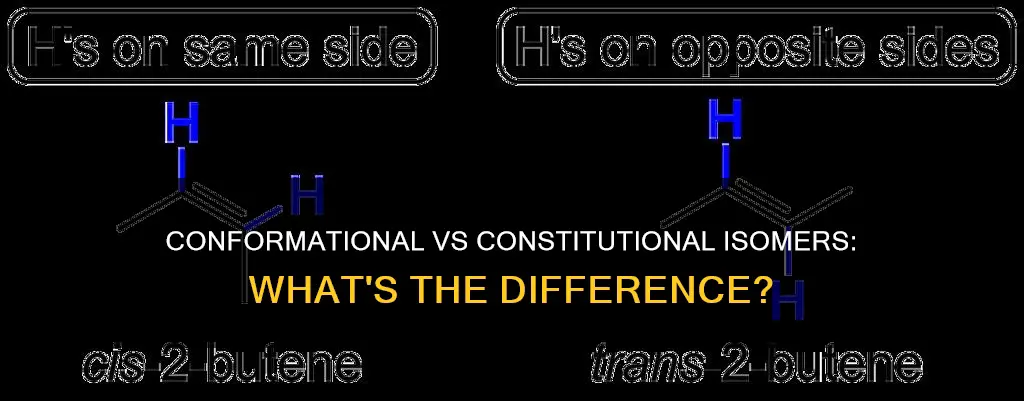
The prefixes "cis" and "trans" are used to distinguish between geometric isomers. The cis-stereoisomer has both non-hydrogen atoms on the same side of the double bond. Whereas, the trans-stereoisomer has the non-hydrogen atoms across the double bond. This small difference may seem insignificant, but geometric isomers are different chemical compounds with different physical properties.
Conformational isomers are a subset of stereoisomers. Stereoisomers have identical molecular formulas and arrangements of atoms. They differ from each other only in the spatial orientation of groups in the molecule. For organic chemistry, there are several types of stereoisomers: enantiomers, diastereomers, geometric isomers, and conformers.
Who Drafted the Constitution?
You may want to see also

Constitutional isomers are also known as structural isomers
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different structures or arrangements. This means that constitutional isomers have the same chemical formula but differ in the bonding arrangement or connectivity of atoms within the molecule. In other words, the atoms in constitutional isomers are the same, but the bonds between them are different. For example, butane (a four-carbon chain) and 2-methylpropane (a one-carbon branch from a three-carbon chain) are constitutional isomers with the same chemical formula C4H10, but they have different bonding patterns. Cyclohexane and methylcyclopentane are another example of constitutional isomers where the atoms are connected differently.
Constitutional isomers are different from conformational isomers, which have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. Conformational isomers are essentially the same molecule but with different forms that can interconvert by rotation around single bonds. For instance, butane can have conformational isomers like staggered and eclipsed forms.
It is important to distinguish between structural differences and spatial differences when comparing compounds. While constitutional isomers have different bonding patterns, stereoisomers or spatial isomers have the same structural connections but differ in the spatial orientation of groups in the molecule. Stereoisomers include conformers, which show the same compound with different carbon-carbon single bond rotations. Geometric isomers, also known as cis and trans isomers, are another type of stereoisomer where the rigidity of the double bond creates a line of reference for spatial orientation.
In organic chemistry, molecules that are considered "identical twins" are not isomers but are instead identified as identical copies of the same molecule. This distinction is made when molecules can be superimposed on each other through rotational changes or through the rotation of the molecule itself. Constitutional isomers, therefore, differ in their bonding arrangements, while conformational isomers differ in their spatial orientation due to rotations around single bonds.
Executive Branch: Its Definition and Significance
You may want to see also
Frequently asked questions
Isomers are compounds with the same molecular formula but different structures or arrangements.
Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of atoms within the molecule. This means the atoms are connected in different ways, leading to different structural formulas.
Conformational isomers have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around a single bond. These are different forms of the same molecule that can interconvert by rotation around single bonds.
Constitutional isomers have different molecular formulas and different connectivity, while conformational isomers have the same molecular formula and connectivity but differ in rotations about single bonds.


























