Matter is a term that has been contemplated by scientists and philosophers for centuries. In physics, matter is defined as baryons and leptons, which can be further broken down into quarks and electrons. These elementary particles combine to form atoms, which are the building blocks of molecules and, ultimately, the bulk matter of everyday objects. The state of matter can be changed by altering temperature or pressure, resulting in solids, liquids, gases, and plasma as the four natural states of matter. The transformation of matter into energy, as described by Einstein's theory of relativity, is a key concept in understanding the relationship between matter and energy.
| Characteristics | Values |
|---|---|
| Definition | A substance made up of various types of particles that occupies physical space and has inertia |
| Composition | Predominantly made up of atoms consisting of protons, neutrons, and electrons |
| States | Solid, liquid, gas, plasma, foam, clusters |
| Physical characteristics | Color, mass, volume |
| Chemical characteristics | How one type of matter interacts with another type of matter |
| Universal properties | Inertia, gravitational mass |
Explore related products
What You'll Learn

Matter is a substance with mass, volume and inertia
Matter is made up of various types of particles, including electrons, protons and neutrons, which form atoms. These atoms can then combine to form molecules. Atoms and molecules in different elements can also join together to form compounds. For example, sodium and chlorine, two highly poisonous elements, combine to form common salt (sodium chloride), which is harmless to humans.
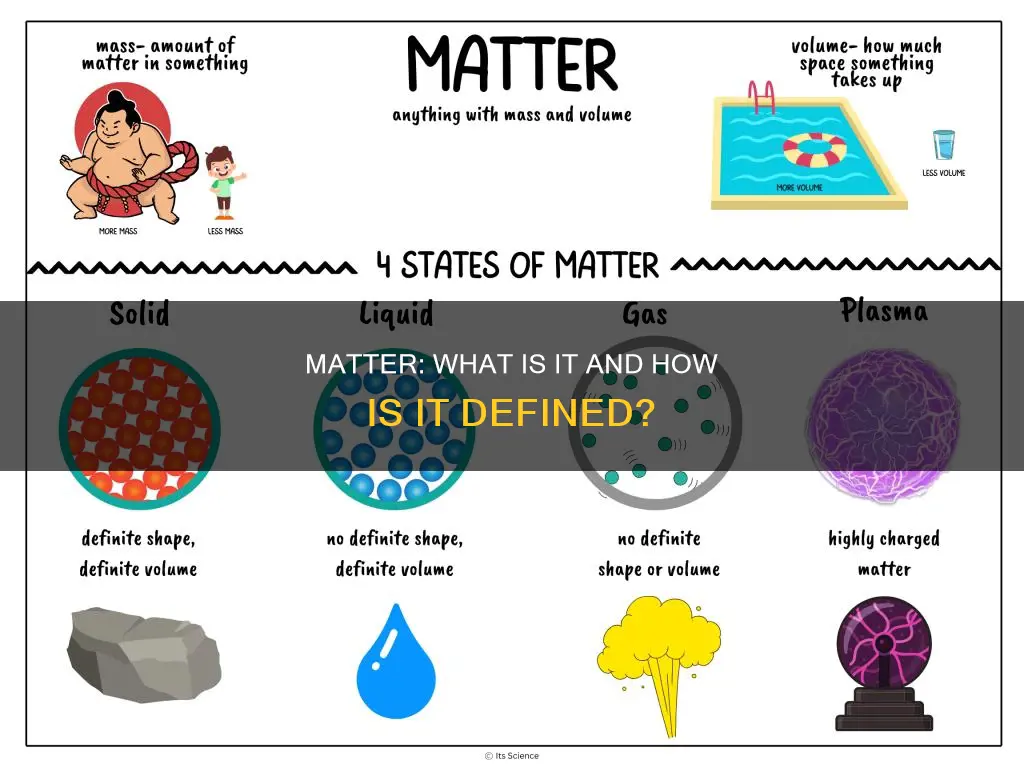
Matter can exist in different states, such as solids, liquids and gases, depending on temperature and other conditions. For instance, at ordinary temperatures, gold is a solid, water is a liquid, and nitrogen is a gas. These states can be further categorised into subgroups, such as metallic or ionic solids.
Matter can also be categorised based on its physical and chemical characteristics, such as colour or mass, and how it interacts with other types of matter.
At the most fundamental level, matter is composed of elementary particles known as quarks and leptons. Quarks combine to form protons and neutrons, which, along with electrons, make up the atoms of elements in the periodic table.
Ordinary matter, composed of quarks and leptons, makes up about 4% of the energy of the observable universe. The remaining energy is theorised to be composed of exotic forms, such as dark matter and dark energy.
Amendments to the Constitution: A Historical Overview
You may want to see also

It occupies space and is made of particles
Matter is a substance that occupies space and is made of particles. It is a general term used to describe any physical substance, and it constitutes the observable universe. Matter can be categorised into solids, liquids, and gases based on certain characteristics. For instance, solids hold their shape, liquids take on the shape of their container, and gases fill their entire container.
Matter is made up of various types of particles, each with a specific mass and size. The most familiar examples of these particles are electrons, protons, and neutrons. These particles combine to form atoms, which are the building blocks of matter. Atoms may further combine to form molecules, such as the water molecule (H2O).
At its most fundamental level, matter is composed of elementary particles known as quarks and leptons. Quarks combine to form protons and neutrons, which, along with electrons, make up atoms of elements such as hydrogen, oxygen, and iron. These atoms and molecules then come together in large groups to form the bulk matter of everyday objects.
Matter exhibits both wave-like and particle-like properties, a concept known as wave-particle duality. It also shares the fundamental property of inertia, which prevents a material body from instantly changing its state of rest or motion. This mass and resistance to change in motion are measurable, with heavier objects having greater mass and being more challenging to set in motion.
Matter can exist in different states, and substances can transition between these states in response to changes in temperature or pressure. For example, when heat is applied to a solid, its particles vibrate rapidly and move apart, causing it to melt. Additionally, matter can be changed from one state to another by physical or chemical means.
Exploring the National Constitution Center: A Half-Day Visit
You may want to see also

Matter is made up of atoms, which are made of protons, neutrons and electrons
Matter is a broad term that describes any substance or material that occupies physical space and has mass. It is a fundamental concept in science, encompassing the building blocks of our physical world. At the most basic level, matter is made up of atoms, which are the fundamental units of elements listed in the periodic table. These atoms, in turn, consist of protons, neutrons, and electrons, each contributing to the overall mass of the atom.
Protons and neutrons are found in the nucleus, the central region of the atom. Protons carry a positive charge, attracting the negatively charged electrons that orbit the nucleus in specific pathways, known as orbitals. Neutrons, on the other hand, are neutrally charged, helping to balance the charges within the atom. The number of protons in an atom determines the type of element it represents, as different elements have distinct numbers of protons.
The combination of these subatomic particles forms the basis of the atom's structure and properties. Protons and neutrons are themselves composed of even smaller particles called quarks, which are bound together by forces described in quantum chromodynamics. Quarks come in several types, or "flavours," and their combinations within protons and neutrons contribute to the unique characteristics of each atom.
Electrons, while also considered elementary particles, are classified as leptons. They play a crucial role in the chemical behaviour of atoms. The arrangement of electrons in their orbitals determines how atoms interact and bond with each other to form molecules. This process gives rise to the diverse compounds and substances that make up the bulk of matter we encounter daily.
The understanding of matter and its constituents has evolved over time, with contributions from scientists such as Newton, Aristotle, and Einstein. Matter can exist in different states, such as solids, liquids, and gases, depending on temperature and pressure conditions. Furthermore, the principles of wave-particle duality highlight that matter exhibits both wave-like and particle-like behaviours, showcasing the complexity of this seemingly simple concept.
Ohio vs US Constitution: What's the Difference?
You may want to see also
Explore related products

Matter can be changed from one state to another by physical or chemical changes
Matter is a general term for any substance or material that occupies space and has mass and volume. It constitutes the observable universe and forms the basis of all objective phenomena. Matter can exist in various states, commonly recognized as solids, liquids, and gases (or plasma). Importantly, matter can change from one state to another through physical or chemical changes.
Physical changes occur when the shape, size, or state of matter changes, but the substance itself remains essentially the same. For example, when a carrot is chopped up or ice melts into water, the matter has undergone a physical change. In these cases, the molecules of the substance do not change; only the appearance or state of the matter is altered.
Chemical changes, on the other hand, involve the breaking and reforming of molecules in new and different combinations. When one or more substances are combined through chemical reactions, they may create an entirely new substance with distinct properties. For instance, pure water is composed of two hydrogen atoms bonded to a single oxygen atom (H2O), but when water boils, it transforms into a gaseous state, like steam. This process, called vaporization, demonstrates how a liquid can change into a gas through the application of heat energy.
The transformation of matter between states is influenced by temperature and pressure conditions. For instance, when solids are heated, they melt into liquids upon reaching their melting point. If further heat is applied, the liquid can boil and transition into a gas at its boiling point. Notably, some solids can bypass the liquid state and directly change into gases through a process called sublimation, as seen with dry ice. Similarly, gases can transform directly into solids. These transitions between states demonstrate the dynamic nature of matter and its responsiveness to energy changes in the surrounding environment.
Matter, at its most fundamental level, is composed of elementary particles known as quarks and leptons. These combine to form protons, neutrons, and electrons, which constitute atoms of elements like hydrogen, oxygen, and iron. Atoms can further combine to create molecules, such as the water molecule (H2O). Thus, matter can be understood as a hierarchical structure, with particles combining to form increasingly complex substances that make up the world around us.
The Republic: A Constitutional Inquiry
You may want to see also

Matter and energy can be converted into each other
Matter is a general term for any physical substance that occupies space and has mass. It is composed of atoms, which are made up of protons, neutrons, and electrons. At the most fundamental level, matter is made up of quarks and leptons, which are known as elementary particles.
Matter can exist in various states, such as solids, liquids, and gases, depending on temperature and pressure conditions. For instance, at ordinary temperatures, gold is a solid, water is a liquid, and nitrogen is a gas. These states can be further categorized into subgroups, such as solids with crystalline or amorphous structures.
Matter and energy are intimately related and can be converted into each other. This relationship is described by Albert Einstein's famous equation, E = mc^2, where E represents energy, m represents mass, and c represents the speed of light. This equation demonstrates that mass and energy are interchangeable.
One example of converting energy into matter is by accelerating atoms to near the speed of light and then colliding them. This process results in the creation of new atoms, with some of the energy used in the acceleration being converted into new matter. Another method involves accelerating electrons just below the speed of light and smashing them into a slab of gold, creating a beam of intense light. This light, when directed into a hollow gold shell, can produce electrons and positrons, which are antimatter particles.
Additionally, during nuclear fission, matter is converted into energy. For instance, when the nucleus of a heavy element like uranium splits into two fragments, the mass difference is released as energy. This process showcases how matter and energy can be interchanged, providing evidence for Einstein's theory of special relativity.
Exploring Beto O'Rourke's Radical Constitution Suggestion
You may want to see also
Frequently asked questions
Matter is a general term describing any physical substance that takes up space and has mass. It is composed predominantly of atoms consisting of protons, neutrons, and electrons.
The four natural states of matter are solid, liquid, gas, and plasma. However, there are other states of matter that can be manufactured in a laboratory under extreme conditions, such as fermionic condensates and time crystals.
The quark-lepton definition of ordinary matter identifies the elementary building blocks of matter and includes composites made from constituents like atoms and molecules. These composites contain an interaction energy that holds the constituents together.
Matter and energy can be converted into each other. This is demonstrated by Einstein's theory of special relativity, which shows that matter (as mass) and energy are interchangeable according to the equation E = mc^2, where E is energy, m is mass, and c is the speed of light.