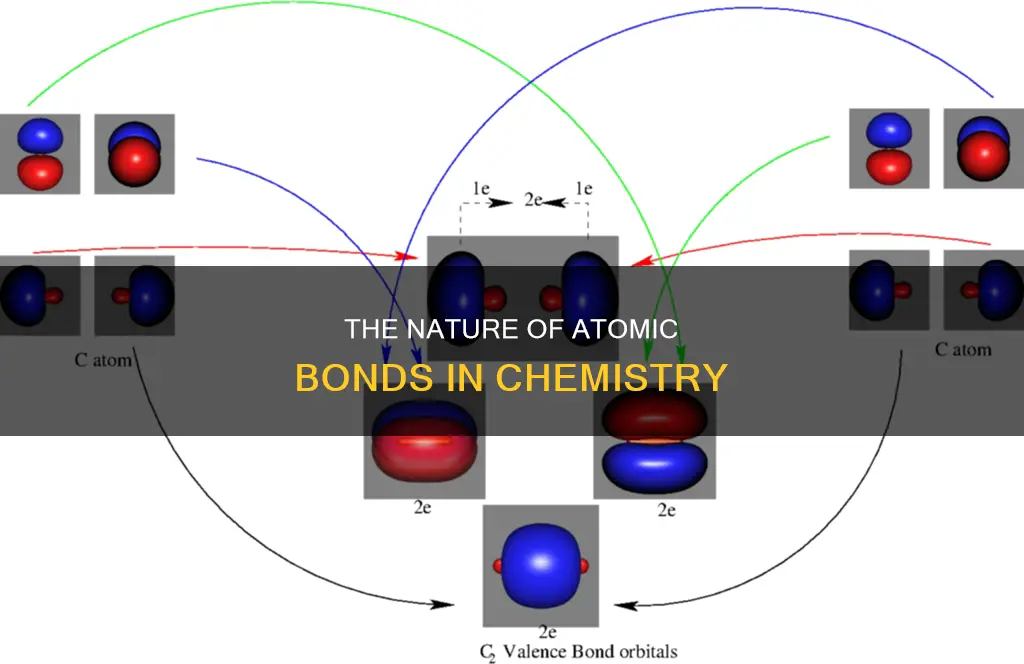
Valence bond theory (VBT) is a theory that describes chemical bonding and the formation of covalent bonds between atoms. It was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London to explain the formation of covalent bonds between two hydrogen atoms using the Schrodinger wave equation. VBT focuses on the concepts of electronic configuration, atomic orbitals, and their overlapping, which leads to the formation of chemical bonds. The theory also explains the electronic structure of molecules formed by this overlapping of atomic orbitals. The energy of the system depends on the degree of overlap between atomic orbitals, and the stability of the resulting molecule is influenced by the electron density in the region between the bonding atoms.
| Characteristics | Values |
|---|---|
| Atomic orbitals | Overlapping atomic orbitals of the participating atoms form a chemical bond |
| Electron configuration | The nucleus of one atom in a molecule is attracted to the electrons of the other atoms |
| Hybridization | Atomic orbitals combine to form new orbitals that better match the geometry of molecules |
| Types of bonds | Sigma and Pi bonds are the two types of overlapping orbitals |
| Sigma bonds | Formed from the head-to-head overlapping of atomic orbitals |
| Pi bonds | Formed from the side-by-side overlapping of atomic orbitals |
| Energy | The energy of the system depends on how much the orbitals overlap |
| Stability | The stability of the molecule increases as the electron density in the area between the two bonding atoms increases |
| Bond distance | At a specific distance, the energy reaches its lowest (most stable) value, this is the optimum distance between the two bonded nuclei |
Explore related products
What You'll Learn

Atomic orbitals and their overlapping
Valence bond theory (VBT) describes chemical bonding and the formation of chemical bonds between atoms. It was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London, and it focuses on the concepts of electronic configuration, atomic orbitals, and their overlapping.
Atomic orbitals are the spaces around an atom's nucleus where electrons are likely to be found. When two atoms come together to form a bond, their orbitals may overlap. This is known as orbital overlap, and it is a crucial concept in understanding chemical bonding. The greater the overlap between orbitals, the stronger the resulting bond. This is because, as orbitals overlap, the electron density in the region between the atoms increases, leading to increased stability in the molecule.
There are two main types of orbital overlaps: positive and negative. Positive overlap occurs when the phases of the interacting orbitals are the same, resulting in bond formation. On the other hand, when the phases of the interacting orbitals are opposite, the overlap is negative, and a bond is not formed. Additionally, zero overlap can occur when orbitals do not overlap at all or do not overlap efficiently, also resulting in no bond formation.
The type of atomic orbitals involved and the way they overlap determine the type of chemical bond formed. For example, sigma (σ) bonds are formed when orbitals overlap head-on or end-to-end along the internuclear axis, resulting in stronger bonds. On the other hand, pi (π) bonds are formed through sidewise overlapping, where the axes of the atoms remain parallel to each other and perpendicular to the internuclear axis. Pi bonds are weaker than sigma bonds because the overlap occurs to a lesser extent.
The valence bond theory also considers the concept of hybridization, which describes how atomic orbitals combine to form new orbitals that better match the geometry of molecules. For instance, in methane (CH4), the carbon atom undergoes sp3 hybridization, forming four equivalent orbitals that result in a tetrahedral shape.
While VBT provides valuable insights into chemical bonding, it has limitations. It assumes that all bonds are localized, formed by the donation of an electron from each atom, which is not always the case. Additionally, it struggles to explain certain complex bonding situations and fails to account for the tetravalency of carbon. Molecular orbital theory (MO), which considers delocalized electrons and provides better predictions of molecular properties, is often preferred for more intricate molecular geometries.
Exploring Police Powers: Constitutional Clause or Omission?
You may want to see also

Hybridization of atomic orbitals
Valence bond theory (VBT) is a theory that describes chemical bonding. It states that the overlap of incompletely filled atomic orbitals leads to the formation of a chemical bond between two atoms. The unpaired electrons are shared and a hybrid orbital is formed. The hybridization of atomic orbitals is a model that describes how atomic orbitals combine to form new orbitals that better match the geometry of molecules. Atomic orbitals that are similar in energy combine to make hybrid orbitals. For example, the carbon in methane (CH4) undergoes sp3 hybridization to form four equivalent orbitals, resulting in a tetrahedral shape.
Different types of hybridization, such as sp, sp2, and sp3, correspond to specific molecular geometries (linear, trigonal planar, and tetrahedral), influencing the bond angles observed in molecules. Hybrid orbitals provide additional directionality to sigma bonds, accurately explaining molecular geometries. The valence bond theory, along with the hybrid orbital concept, does a very good job of describing double-bonded compounds such as ethene. Three atomic orbitals on each carbon—the 2s, 2px, and 2py orbitals—combine to form three sp2 hybrids, leaving the 2pz orbital unhybridized.
Hybridization was introduced to explain molecular structure when the valence bond theory failed to correctly predict them. It is experimentally observed that bond angles in organic compounds are close to 109°, 120°, or 180°. According to the Valence Shell Electron Pair Repulsion (VSEPR) theory, electron pairs repel each other, and the bonds and lone pairs around a central atom are generally separated by the largest possible angles. The number of hybrid orbitals formed is equal to the number of atomic orbitals mixed. It is not necessary that all the half-filled orbitals must participate in hybridization. Even completely filled orbitals with slightly different energies can also participate.
Judicial Duty: Constitution Compliance or Judicial Independence?
You may want to see also

Covalent bond formation
Valence bond theory (VBT) is a theory that describes chemical bonding. It was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London and builds on the work of G. N. Lewis, who proposed in 1916 that a chemical bond forms when electrons are shared between two atoms.
VBT states that covalent bonds are formed when two half-filled valence orbitals from different atoms overlap. This overlap results in an increase in electron density in the region between the atoms, leading to greater stability in the resulting molecule. The unpaired electrons are shared, forming a hybrid orbital. The nucleus of one atom is attracted to the electrons of the other atom, and vice versa. The energy of the system depends on the amount of orbital overlap, with the optimum distance between the two bonded nuclei resulting in the lowest energy configuration and a stable bond.
Sigma bonds are formed when orbitals overlap head-to-head, while pi bonds are formed through side-by-side or parallel overlap. The type of overlap depends on the atoms involved and influences the strength of the bond. For example, sigma bonds are stronger than pi bonds due to the greater overlap of orbitals in sigma bonds.
VBT also considers hybridization, where atomic orbitals combine to form new orbitals that better match the geometry of molecules. For instance, in methane (CH4), the carbon undergoes sp3 hybridization, resulting in a tetrahedral shape.
While VBT provides valuable insights into chemical bonding, it has limitations. It assumes that all bonds are localized, which is not always the case, and it fails to explain certain phenomena, such as the tetravalency of carbon. Molecular orbital theory (MO) is often considered more accurate in predicting molecular properties, but VBT offers a more intuitive understanding of the formation and breaking of bonds during chemical reactions.
Southern Secession: Constitutional Crisis or Planned Strategy?
You may want to see also
Explore related products
$109 $135.95
$21.95 $21.95

Sigma and pi bonds
Valence bond theory (VBT) describes the formation of chemical bonds between atoms. It was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London, building on the work of G. N. Lewis, Charles Rugeley Bury, and Walther Kossel. VBT holds that covalent bonds are formed when two half-filled valence orbitals from different atoms overlap, with unpaired electrons shared between them. This overlap increases the electron density between the atoms, resulting in a stable molecule.
Pi bonds, on the other hand, are formed when the unhybridized p-orbitals of atoms overlap side-by-side or in a parallel fashion. This overlap results in electron density concentrated above and below the plane of the nuclei of the bonding atoms. Pi bonds are formed outside of the established sigma bond and prevent the bond from rotating. Double bonds are comprised of one sigma and one pi bond, while triple bonds consist of one sigma and two pi bonds.
The distinction between sigma and pi bonds is important in understanding the properties of molecules. For instance, single sigma bonds allow free rotation around the axis of the two nuclei, while the presence of pi bonds restricts this rotation. Additionally, the combination of sigma and pi bonds helps explain the existence of double and triple bonds in organic chemistry.
California's Unlicensed Medical Practice: What You Need to Know
You may want to see also

Electron density and stability
Valence bond theory (VBT) is a theory that describes chemical bonding. It states that the overlap of incompletely filled atomic orbitals leads to the formation of a chemical bond between two atoms. The unpaired electrons are shared and a hybrid orbital is formed. The overlap of orbitals results in an increase in electron density in the area between the two bonding atoms, thereby increasing the stability of the resulting molecule.
The valence bond theory was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London to address the issues with the valence shell electron-pair repulsion (VSEPR) theory. The VSEPR theory had limited applications and failed to predict the geometry of complex molecules. VBT focuses on the concepts of electronic configuration, atomic orbitals, and the hybridization of these atomic orbitals. It also explains the electronic structure of molecules formed by the overlapping of atomic orbitals.
According to VBT, a covalent bond is formed when two conditions are met: (1) an orbital on one atom overlaps an orbital on a second atom, and (2) the single electrons in each orbital combine to form an electron pair. The mutual attraction between this negatively charged electron pair and the two atoms' positively charged nuclei creates a covalent bond. The strength of a covalent bond depends on the extent of overlap of the orbitals involved. Orbitals that overlap extensively form stronger bonds than those with less overlap.
The formation of sigma (σ) and pi (π) bonds is explained by the valence bond theory. Sigma bonds occur when the orbitals of two shared electrons overlap head-to-head, with the electron density concentrated in the region along the internuclear axis. Pi bonds, on the other hand, are formed from the side-by-side overlap of two p orbitals.
The presence of many unpaired electrons in the valence shell of an atom enables it to form multiple bonds with other atoms. However, it is important to note that valence bond theory assumes that all bonds are localized, which is not always the case as many atoms also bond using delocalized electrons. Additionally, VBT fails to explain certain phenomena, such as the tetravalency of carbon and the energies of electrons.
Emails from My Landlord: Harassment or Not?
You may want to see also
Frequently asked questions
Valence bond theory (VBT) describes chemical bonding and the formation of covalent bonds between atoms. It was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London.
According to VBT, chemical bonds are formed when atomic orbitals of participating atoms overlap, resulting in the sharing of unpaired electrons and the formation of a hybrid orbital. This overlap increases the electron density between the atoms, leading to increased stability of the resulting molecule.
VBT assumes that all bonds are localized between two atoms, which is not always the case as some atoms use delocalized electrons for bonding. It also fails to explain the tetravalency of carbon and does not provide insights into the energies of electrons. VBT is typically applied to relatively small molecules.