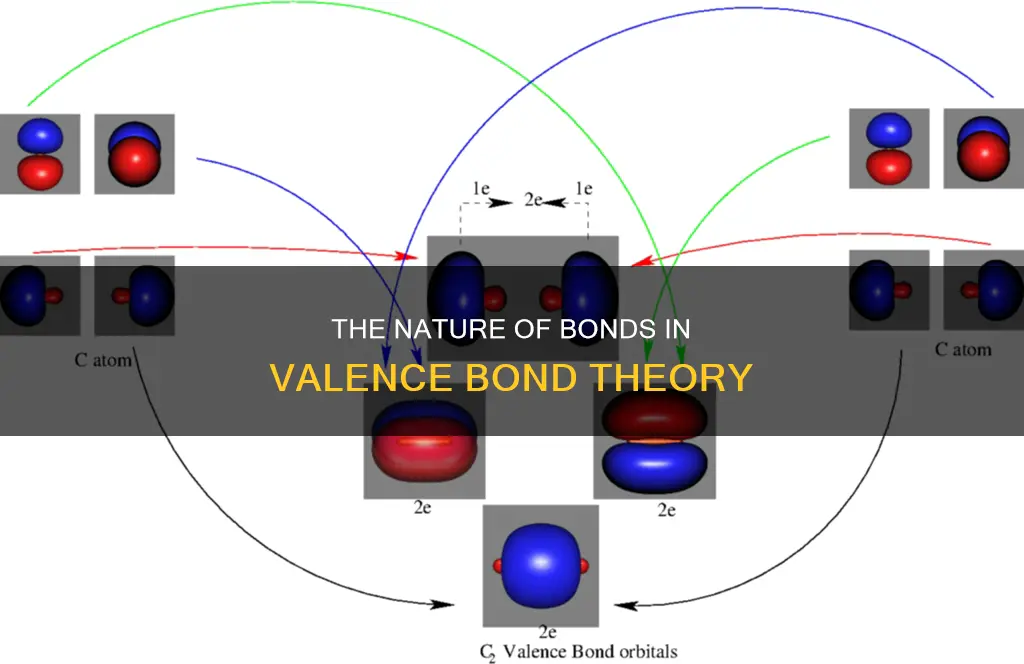
Valence Bond Theory (VBT) is one of two basic theories that use quantum mechanics to explain chemical bonding. It was developed by German physicists Walter Heitler and Fritz London, who used the Schrodinger wave equation to explain the formation of a covalent bond between two hydrogen atoms. VBT assumes that all bonds are localized and formed between two atoms by the donation of an electron from each atom. It predicts covalent bond formation between atoms when they have half-filled valence atomic orbitals, each containing a single unpaired electron. These atomic orbitals overlap, so electrons are most likely to be within the bond region. The strength of a covalent bond depends on the extent of overlap of the orbitals involved.
| Characteristics | Values |
|---|---|
| Focus | Concepts of electronic configuration, atomic orbitals, and their overlapping |
| Formation of covalent bonds | Overlapping of half-filled valence atomic orbitals of each atom |
| Electron pair formation | Single electrons in each orbital combine to form an electron pair |
| Electron density | Increases in the area between the two bonding atoms |
| Bond strength | Depends on the extent of overlap of the orbitals involved |
| Bond types | Sigma and Pi bonds |
| Sigma bonds | Formed by head-to-head overlap of orbitals |
| Pi bonds | Formed by parallel overlap of orbitals |
| Hybridization | Orbitals combine to form new orbitals that better match the geometry of molecules |
| Quantitative interpretation | Unable to provide a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds |
Explore related products

Hybridization
The type of hybrid orbitals formed depends on the electron-pair geometry as predicted by the VSEPR theory. Hybrid orbitals overlap to form σ (sigma) bonds, while unhybridized orbitals overlap to form π (pi) bonds. For instance, in a gaseous BeCl2 molecule, two of the beryllium atom's four valence orbitals mix to yield two sp hybrid orbitals, resulting in a linear geometry. Similarly, in boron atoms with three regions of electron density, three of the orbitals hybridize to create three sp2 orbitals and one unhybridized 2p orbital. These hybrid orbitals then overlap with hydrogen atoms to form single bonds in BH3.
Valence bond theory, along with molecular orbital (MO) theory, was developed to use quantum mechanics to explain chemical bonding. It focuses on how atomic orbitals of dissociated atoms combine to form chemical bonds when a molecule is formed. According to VBT, chemical bonds are formed by the overlap of half-filled valence atomic orbitals, leading to increased electron density and stability in the resulting molecule. This theory also explains the electronic structure of molecules formed by orbital overlapping.
The Constitution: Ensuring Fair Trials
You may want to see also

Atomic orbitals
Valence bond theory (VBT) is one of the two basic theories, along with molecular orbital (MO) theory, that uses quantum mechanics to explain chemical bonding. VBT was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London. It explains the formation of a covalent bond between two hydrogen atoms using the Schrodinger wave equation.
VBT assumes that all bonds are localized bonds formed between two atoms by the donation of an electron from each atom. This assumption is not always valid, as many atoms bond using delocalized electrons. According to VBT, covalent bonds are formed when two half-filled valence orbitals from different atoms overlap. This overlap increases the electron density in the area between the atoms, increasing the stability of the resulting molecule.
Hybridization is a concept related to atomic orbitals that is important in VBT. It describes how atomic orbitals of similar energy combine to form new orbitals, called hybrid orbitals, that better match the geometry of molecules. For example, in methane (CH4), the carbon atom undergoes sp3 hybridization, resulting in four equivalent orbitals that give methane its tetrahedral shape. Hybrid orbitals influence bond angles and provide directionality to sigma bonds, contributing to our understanding of molecular geometries.
In summary, atomic orbitals and their overlapping and hybridization are fundamental concepts in VBT. By considering the interaction of atomic orbitals, VBT provides insights into the formation of chemical bonds and the electronic structure of molecules.
Safeguarding Rights: US Constitution's Limits on Majority Rule
You may want to see also

Overlapping orbitals
Valence Bond Theory (VBT) is one of the two basic theories that use quantum mechanics to explain chemical bonding. It was put forth by German physicists Walter Heinrich Heitler and Fritz Wolfgang London. The theory focuses on electronic configuration, atomic orbitals, and the hybridization of atomic orbitals.
According to VBT, chemical bonds are formed by the overlapping of atomic orbitals. This occurs when two valence orbitals (half-filled) from different atoms overlap, and the single electrons in each orbital combine to form an electron pair. This mutual attraction between the negatively charged electron pair and the positively charged nuclei of the atoms forms a covalent bond. The strength of the bond depends on the extent of the overlap. Orbitals that overlap extensively form stronger bonds than those with less overlap.
The overlapping of orbitals also increases the electron density in the area between the bonding atoms, increasing the stability of the resulting molecule. This is known as the maximum overlap condition and can explain the formation of covalent bonds in several molecules. For example, the difference in the length and strength of chemical bonds in H2 and F2 molecules can be explained by the difference in the overlapping orbitals.
Sigma (σ) bonds and pi (π) bonds differ in the pattern of atomic orbital overlap. Sigma bonds are formed from the head-to-head or end-to-end overlap of two atomic orbitals, such as two s orbitals, an s orbital and a p orbital, or two p orbitals. Pi bonds, on the other hand, are formed from the side-by-side or parallel overlap of two p orbitals.
VBT also describes how atomic orbitals combine to form new orbitals that better match the geometry of molecules. This process is called hybridization and results in orbitals of definite geometry, such as octahedral, tetrahedral, or square planar. These hybrid orbitals can then overlap with other orbitals to form chemical bonds.
The Two-Party System: Is It Constitutional?
You may want to see also
Explore related products
$109 $135.95

Electron pairs
Valence bond theory (VBT) is one of the two basic theories that use quantum mechanics to explain chemical bonding. It focuses on how the atomic orbitals of the dissociated atoms combine to form chemical bonds when a molecule is formed.
VBT theory assumes that all bonds are localized bonds formed between two atoms by the donation of an electron from each atom. This assumption is not always valid, as many atoms bond using delocalized electrons. According to VBT, covalent bonds are formed when two half-filled valence orbitals from different atoms overlap. This overlap increases the electron density in the region between the atoms, increasing the stability of the resulting molecule.
The valence shell electron pair repulsion (VSEPR) theory, also known as Vesper theory, is a model used in VBT to predict the 3D molecular geometry of a molecule or ion. It assumes that electron pairs will arrange themselves to minimize repulsion effects from one another, resulting in electron pairs being as far apart as possible. The VSEPR theory helps determine the spatial orientation of molecules, which is crucial for understanding their reactivity and properties.
The VSEPR model considers both bonding pairs (those involved in chemical bonds) and lone pairs (non-bonding pairs) of electrons. Double and triple bonds are counted as one electron group because they occupy one region of space around the central atom. The number of electron groups, including lone pairs and bonds, is essential for predicting molecular geometry using VSEPR theory.
In summary, electron pairs play a crucial role in VBT by influencing the formation of chemical bonds and determining the 3D molecular geometry through the VSEPR theory.
The US Constitution: Foundation of a Nation's Laws and Values
You may want to see also

Covalent bonds
Covalent bonding occurs when non-metal atoms share their valence electrons to gain more stability, which is gained by forming a full electron shell. Non-metals will readily form covalent bonds with other non-metals to obtain stability. Atoms can form anywhere between one to three covalent bonds with other non-metals depending on how many valence electrons they possess.
The idea of shared electron pairs was introduced in 1916 by the American chemist G.N. Lewis, who described the formation of such bonds as resulting from the tendencies of certain atoms to combine with one another in order for both to have the electronic structure of a corresponding noble-gas atom. In 1919, Irving Langmuir referred to the term "covalence" in an article in the Journal of the American Chemical Society.
In 1927, Walter Heitler and Fritz London provided the first successful quantum mechanical explanation of a chemical bond (molecular hydrogen), based on the valence bond model.
The Founding Fathers and the Family Unit
You may want to see also
Frequently asked questions
Valence Bond (VB) Theory is one of the two basic theories, along with Molecular Orbital (MO) Theory, that uses the methods of
Valence bond theory considers that the overlapping atomic orbitals of the participating atoms form a chemical bond. The mutual attraction between the negatively charged electron pair and the two atoms' positively charged nuclei physically link the two atoms through a force defined as a covalent bond.
Valence bond theory assumes that all bonds are localized bonds formed between two atoms by the donation of an electron from each atom. It also assumes that electrons are localized in specific areas.
The valence bond theory has important applications in explaining the formation of covalent bonds in several molecules. For example, the difference in the length and strength of the chemical bonds in H2 and F2 molecules can be explained by the difference in the overlapping orbitals in these molecules.
The limitations of valence bond theory include its failure to explain the tetravalency of carbon, its assumption that electrons are localized in specific areas, and its inability to provide a quantitative interpretation of the thermodynamic or kinetic stabilities of coordination compounds.











































