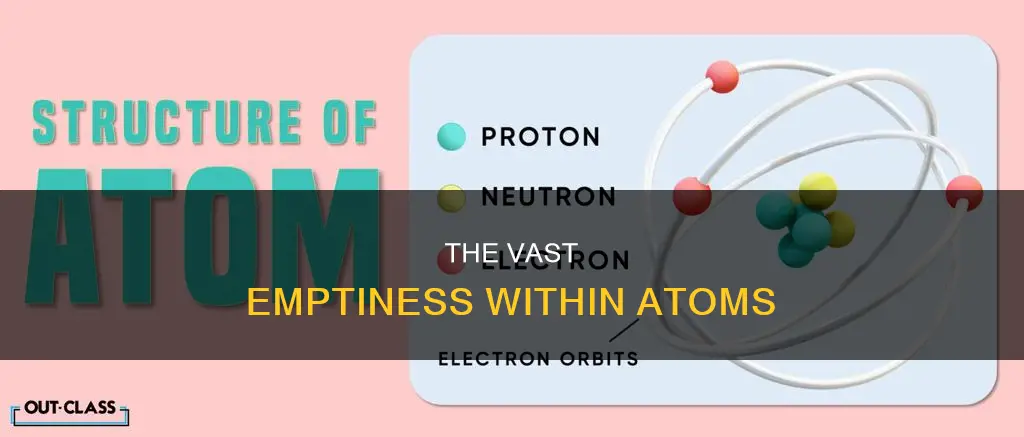
Atoms are the smallest unit of matter and are composed of subatomic particles: protons, neutrons, and electrons. While the majority of an atom's mass is concentrated in its nucleus, most of its volume is comprised of empty space. This space is occupied by the electron cloud, which refers to the area between the nucleus and the orbitals where electrons are most likely to be found. The nucleus is incredibly small, with a diameter of approximately 10^-15 meters, while the diameter of an atom is around 10^-10 meters. This means that the nucleus takes up only a tiny fraction of the atom's total volume, leaving the rest to be filled by the electron cloud.
| Characteristics | Values |
|---|---|
| Most of the volume of an atom | Empty space |
| Most of the mass of an atom | Nucleus |
| Protons | Positively charged particles |
| Neutrons | No charge, slightly more massive than protons |
| Electrons | Negatively charged particles |
| Electron cloud | Area between the nucleus and orbitals where the probability of finding electrons is maximum |
Explore related products
What You'll Learn

Most of an atom's volume is empty space
Atoms are composed of three basic types of subatomic particles: protons, neutrons, and electrons. Protons are positively charged ions, electrons are negatively charged ions, and neutrons are neutral subatomic particles. Most of an atom's mass is concentrated in its nucleus, which is made up of protons and neutrons. However, the volume of an atom is largely occupied by electron clouds.
The nucleus of an atom is extremely small compared to the overall size of the atom. It is estimated that the nucleus takes up only about 1 part in 100,000 of the space in an atom. To put it into perspective, if the nucleus were the size of a blueberry, the atom would be about the size of a football stadium.
The electrons in an atom revolve around the nucleus in fixed circular paths called orbits or shells. These electron orbits contribute significantly to the volume of the atom. In fact, most of the volume of an atom is comprised of the space between the nucleus and these electron orbits, often referred to as "empty space."
This concept of empty space within an atom is intriguing, especially considering that there is no true "empty" space in the universe. Nonetheless, the term "empty space" in this context refers to the regions of an atom where the probability of finding electrons is relatively low compared to the regions closer to the nucleus.
It is important to note that the structure of an atom can vary depending on the element. For example, a hydrogen atom typically contains one proton but no neutrons, making it an exception to the typical composition of an atomic nucleus.
Executive Branch Leaders: Their Roles and Responsibilities
You may want to see also

The nucleus is very small
The atom is the smallest unit of a molecule that retains its chemical properties. While atoms constitute matter, they themselves consist of smaller particles called subatomic particles. These subatomic particles are protons, neutrons, and electrons. Protons are positively charged ions, electrons are negatively charged ions, and neutrons are neutral subatomic particles.
The nucleus of an atom is composed of protons and neutrons and is, therefore, positively charged. The nucleus is where most of an atom's mass is concentrated because protons and neutrons are much heavier than electrons. The diameter of an atom is about 10−10 m, whereas the diameter of the nucleus is about 10−15 m—about 100,000 times smaller. This means that the nucleus takes up only 10−14 metres of the space in the atom, or 1 part in 100,000.
To put this into perspective, if the nucleus were the size of a blueberry, the atom would be about the size of a football stadium. The radius of an atom measures 1-2 Å. Compared to the overall size of the atom, the nucleus is minute and is in the same proportion to the atom as a marble is to a football field.
Despite the small size of the nucleus, virtually all the mass of the atom is concentrated there. This is because the protons are massive, positively charged particles, and the neutrons are slightly more massive and carry no charge.
Presidential Power: Constitutional Threat or Necessary Evolution?
You may want to see also

Protons and neutrons form the nucleus
Atoms are composed of three basic types of subatomic particles: protons, neutrons, and electrons. Protons are positively charged ions, neutrons are neutral and have no charge, and electrons are negatively charged ions. The nucleus of an atom is formed by the protons and neutrons, with the electrons orbiting around the nucleus in a fixed circular path.
The nucleus of an atom is extremely small compared to the overall size of the atom. The diameter of an atom is approximately 10−10 m, while the diameter of the nucleus is about 10−15 m, making the nucleus about 100,000 times smaller than the atom as a whole. To put it into perspective, if the nucleus were the size of a blueberry, the atom would be as big as a football stadium.
Despite the nucleus's minuscule size, it contains the majority of an atom's mass. This is because protons and neutrons are significantly heavier than electrons. The number of particles in the nucleus determines its diameter, which can range from approximately 4 femtometers for a light nucleus like carbon to 15 femtometers for a heavy nucleus like lead.
The mass of an atom is determined by the mass of the nucleus and the electrons. The atomic mass unit (amu) is a unit of measurement used to describe the properties of atoms, and it was originally defined based on hydrogen, the lightest element. However, since 1961, it has been defined regarding the most abundant isotope of carbon, with atoms assigned a mass of exactly 12 amu.
The number of protons in the nucleus, also known as the atomic number, is crucial in identifying the type of atom. For example, an atom with an atomic number of 6 is carbon, while an atom with an atomic number of 92 is uranium. The number of neutrons in the nucleus can vary, and this variation contributes to the different masses of atoms.
Strong Constitution: Foundation for a Healthy, Happy Life
You may want to see also
Explore related products

Electrons are negatively charged
The atom is the smallest unit of a molecule that retains its chemical properties. It is composed of subatomic particles, including electrons, which are negatively charged. Electrons are incredibly small, with a charge of less than 2 x 10^-19 coulombs. They are the lightest charged particles found in nature.
Electrons are one of the fundamental particles that make up an atom, along with protons and neutrons. Protons are positively charged, while neutrons are neutral. These three particles are held together by electrical forces. The positive protons attract the negative electrons, keeping them in orbit around the central nucleus, which consists of protons and neutrons.
The atom is mostly empty space, with the nucleus taking up only a small fraction of its volume. The diameter of an atom is approximately 10^-10 meters, while the diameter of the nucleus is about 100,000 times smaller, at around 10^-15 meters. To put this into perspective, if the nucleus were the size of a blueberry, the atom would be as big as a football stadium.
Electrons are found outside the nucleus, revolving in fixed paths called orbits or shells. These paths are also referred to as electron clouds, and they occupy most of the volume of an atom. The number of electrons in these orbits matches the number of positively charged protons in the nucleus, making the overall atom electrically neutral.
The number of electrons in an atom can vary. For example, a neutral iodine atom has 53 protons and 53 electrons, while a platinum ion can have 74 electrons.
The New York Constitution: Bills of Attainder?
You may want to see also

The atom is electrically neutral
The atom is the smallest unit of any molecule that retains its chemical properties. It is composed of three basic types of subatomic particles: protons, neutrons, and electrons. Protons are positively charged ions, electrons are negatively charged ions, and neutrons are neutral subatomic particles with no charge. The atom is electrically neutral because the number of protons is equal to the number of electrons, meaning the positive charge of protons completely counterbalances the negative charge of the electrons.
The nucleus of an atom is composed of protons and neutrons and contains the majority of an atom's mass. The nucleus is extremely small compared to the overall size of the atom. The diameter of an atom is approximately 10−10 m, while the diameter of the nucleus is about 10−15 m, or about 100,000 times smaller. To put it into perspective, if the nucleus were the size of a blueberry, the atom would be about the size of a football stadium.
The mass of an atom consists of the mass of the nucleus plus the mass of the electrons. The atomic mass unit (amu) is used to measure the extremely small mass of atoms and their constituent particles. The amu was originally defined based on hydrogen, the lightest element, and later in terms of oxygen. Since 1961, it has been defined regarding the most abundant isotope of carbon, with atoms assigned a mass of exactly 12 amu.
Electrons are much lighter than protons and neutrons, and they occupy almost all of an atom's volume. They revolve around the nucleus in fixed circular paths called orbits or shells. These paths are orbital routes that constitute layers, each of which can only hold a certain number of electrons. The atom is held together by the electric forces between the charged particles, with the negatively charged electrons attracted to the positively charged nucleus.
Central Bank Independence: Meaningful Autonomy Explored
You may want to see also
Frequently asked questions
Electrons occupy almost all of an atom's volume.
Electrons are located outside the atom's nucleus, revolving around it in a fixed circular path called orbits.
The path of the electrons is orbital and is often referred to as shells.
The nucleus takes up only about 1 part in 100,000 of the volume of an atom.
The nucleus is about 100,000 times smaller than the atom.