T follicular helper cells (TFH) are a specialized subset of CD4+ T cells that play a significant role in the adaptive immune response, providing critical help to B cells within the germinal centers (GC) of secondary lymphoid organs. TFH cells are defined by their constitutive expression of the B-cell follicle homing receptor CXCR5 and are located in the B-cell zone of secondary lymphoid organs, including the tonsils, spleen, and lymph nodes. They promote B-cell maturation, differentiation, and antibody production. The interaction between TFH and B cells is critical for protective immunity, and a better understanding of this relationship can help improve vaccination strategies and address autoimmune diseases.
| Characteristics | Values |
|---|---|
| Tfh cell location | Secondary lymphoid organs such as lymph nodes, spleen and Peyer's patches |
| Tfh cell type | CD4+ T cells |
| Tfh cell function | Provide help to B cells, supporting the formation of germinal centres, affinity maturation of antibody responses, antibody production, B cell maturation and differentiation |
| Tfh cell markers | CXCR5, PD1, ICOS, Bcl-6 |
| Tfh cell cytokines | IL-21, IL-4 |
| Tfh cell transcription factors | Bcl6, Maf, Ascl2 |
| Tfh cell regulation | CTLA4 and IL-2 pathways, regulatory T cells |
| Tfh cell role in autoimmunity | Increased frequency of Tfh cells is associated with autoimmunity |
Explore related products
What You'll Learn
- T follicular helper cells (TFH) are a specialised subset of CD4+ T cells
- TFH cells are essential B cell-help providers in the formation of germinal centres (GCs)
- TFH cells direct B cells to differentiate into antibody-secreting plasma cells
- TFH cells are defined by expression of the transcription factor B cell lymphoma 6 (Bcl6)
- TFH cells play a critical role in protective immunity, helping B cells produce antibodies against foreign pathogens

T follicular helper cells (TFH) are a specialised subset of CD4+ T cells
TFH cells are defined by their expression of the transcription factor B cell lymphoma 6 (Bcl-6) and cell surface markers such as CXCR5, a chemokine receptor that allows TFH cells to migrate towards the B cell zone. The interaction between TFH and B cells is facilitated by the co-stimulatory molecule CD40, which is expressed on B cells and interacts with CD40 ligand (CD40L) on TFH cells. This interaction provides signals that promote B cell development, proliferation and antibody production. TFH cells also secrete cytokines such as IL-21, which is crucial for B cell proliferation and antibody production.
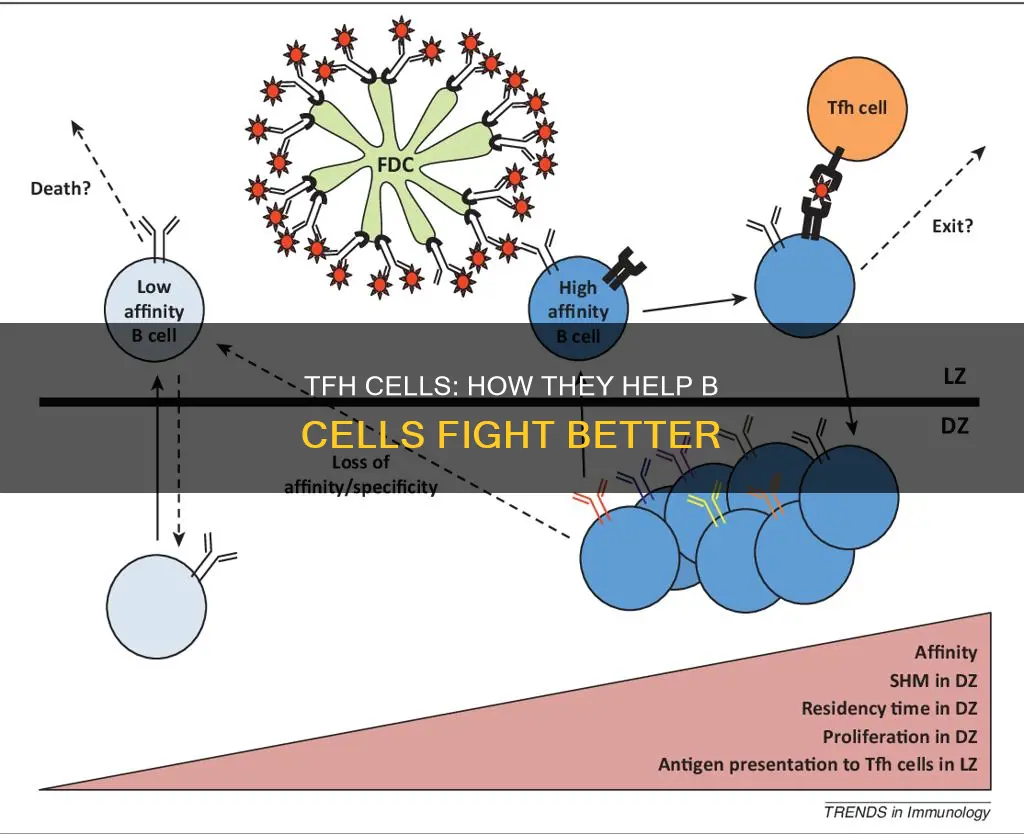
The differentiation of naïve CD4+ T cells into TFH cells is a multi-step process that involves the induction of Bcl-6 expression and the down-regulation of the chemokine receptor CCR7. This allows the developing TFH cells to migrate towards the B cell zone, where they interact with B cells and provide essential help in the formation of germinal centres (GCs). GCs are distinct structures that form within the B cell zones of SLOs during an active immune response. B cells within GCs, known as GC B cells, undergo rapid proliferation and antibody diversification, resulting in the production of various antibodies with greater affinity for their targets.
TFH cells play a critical role in mediating the selection and survival of B cells, which can differentiate into long-lived plasma cells capable of producing high-affinity antibodies or memory B cells that allow for long-lasting antibody production. The dysregulation of TFH cell function can lead to excessive or insufficient antibody production, contributing to autoimmune diseases. Therefore, understanding the biology of TFH cells and their role in immunity and disease is of significant interest for therapeutic purposes and improving vaccination strategies.
Pennsylvania Constitution: Mail-in Voting Legality
You may want to see also

TFH cells are essential B cell-help providers in the formation of germinal centres (GCs)
TFH cells are a specialized subset of CD4+ T cells that play a critical role in protective immunity, helping B cells produce antibodies against foreign pathogens. TFH cells are located in secondary lymphoid organs (SLOs), including the tonsils, spleen, and lymph nodes. These organs contain numerous lymphocytes, separated into defined T and B cell zones.
TFH cells are essential B cell-help providers in the formation of germinal centers (GCs). GCs are anatomic regions in lymphoid organs where activated B cells undergo rapid proliferation and somatic mutation, leading to the differentiation of memory B cells or long-lived plasma cells. TFH cells provide critical helper signals, such as CD40L, that trigger the formation and maintenance of GCs. In the absence of TFH cells, GCs do not form, and antibody defects are observed.
TFH cells express the chemokine receptor CXCR5, which allows them to migrate towards the B cell zone. At the T and B cell zone border, TFH cells interact with activated B cells, providing co-stimulation through the co-stimulatory molecule CD40 and producing cytokines like IL-21. This interaction directs the differentiation of B cells into antibody-secreting plasma cells and memory B cells, allowing for long-lasting antibody production.
The differentiation of TFH cells is a multi-step process, influenced by transcription factors like Bcl-6, and the expression of surface molecules such as CXCR5, PD1, and ICOS. The regulation of TFH cell help is crucial for achieving the goal of GC responses, which is to generate and select GC B cells with higher affinity for pathogens. An improved understanding of TFH biology and its role in diseases may provide valuable insights for therapeutic interventions.
Understanding War Powers: Declarations and Resolutions
You may want to see also

TFH cells direct B cells to differentiate into antibody-secreting plasma cells
T follicular helper cells (TFH) are a specialised subset of CD4+ T cells that play a critical role in protective immunity, helping B cells produce antibodies against foreign pathogens. TFH cells are found in the B cell follicles of secondary lymphoid organs, such as the tonsils, spleen and lymph nodes. They are defined by their expression of the chemokine receptor CXCR5, which allows them to migrate towards the B cell zone.
TFH cells are essential for the formation of germinal centres (GCs), which are structures that form within the B cell zones during an immune response. Within these GCs, B cells differentiate into antibody-secreting plasma cells and memory B cells, allowing for long-lasting antibody production. TFH cells direct this process by providing co-stimulation to the B cells through the interaction of the co-stimulatory molecule CD40 on the B cell with its ligand, CD40L, on the TFH cell. Additionally, TFH cells produce the cytokine IL-21, which drives B cell proliferation and antibody diversification. The production of other cytokines by TFH cells can also influence the type of antibody produced.
The differentiation of B cells into antibody-secreting plasma cells is further facilitated by the expression of CD40L on TFH cells, which stimulates the B cell surface receptor CD40. This interaction leads to the activation of B cells and their differentiation into plasma cells capable of secreting antibodies. The TFH cells formed during the early stages of germinal centre development are known as pre-TFH cells and are found at the border of the T cell zone and B cell follicles.
TFH cells also play a role in mediating the selection and survival of B cells, ensuring that potentially autoimmune-causing mutated B cells are negatively selected against in the germinal centre. This process is not yet fully understood, but it is known that TFH cells can influence the magnitude and quality of the immune response through their production of cytokines. For example, IL-4 secreted by TFH2 cells can promote low-affinity antibodies, while IL-17 produced by TFH17 cells induces antibody isotype class switching.
The proper functioning of TFH cells is crucial for effective immune responses. Dysregulation of TFH cell function has been observed in diseases characterised by excessive or insufficient antibody production, such as systemic lupus erythematosus, rheumatoid arthritis, and common variable immunodeficiency. A better understanding of TFH cells and their role in B cell differentiation could lead to therapeutic advancements in the treatment of such diseases.
Celebrating Spain's Constitution Day: Traditions and Customs
You may want to see also
Explore related products

TFH cells are defined by expression of the transcription factor B cell lymphoma 6 (Bcl6)
T follicular helper cells (TFH) are a specialised subset of CD4+ T cells that play a critical role in protective immunity, helping B cells produce antibodies against foreign pathogens. TFH cells are defined by the expression of the transcription factor B cell lymphoma 6 (Bcl6), which is central to gene regulation in TFH biology, including differentiation and function. Bcl6 is essential for TFH differentiation and has various significant activities, including the inhibition of Prdm1 expression.
Bcl6 is thought to control non-TFH and TFH genes through two modes of action: direct repression and repression-of-repressor mechanisms. By directly binding to and repressing a set of genes for alternative cell fates, cytokines, receptors, and migratory genes, Bcl6 plays a crucial role in TFH cell differentiation. Additionally, Bcl6 indirectly upregulates important functional molecules through repression-of-repressor mechanisms via a set of Bcl6 target transcription factors (Bcl6-r TFs). This includes the repression of Prdm1, Id2, Runt-related transcription factor 2 and 3 (Runx2/3), and Krüppel-like factors.
The transcription factor Bcl6, by serving as an apex of a repressor-of-repressors network, provides insights into TFH cell regulation in both mice and humans. While TFH differentiation and function are conserved across species, differences exist between mice and humans. For instance, IL-6 induces TFH differentiation in mice, but its role in human TFH cells is unclear. Gene-editing technologies, such as CRISPR-Cas9, can be utilised to investigate the roles of transcription factors in TFH differentiation in human CD4+ T cells, offering potential insights into vaccine design and the understanding of relevant diseases.
The precise lineage relationship of TFH cells to other effector CD4+ T cell subsets remains uncertain. However, studies have shown that TFH cells have distinct gene expression profiles, suggesting they are a unique subset. The identification of TFH cell-specific pathogenic changes in autoimmunity is an important area of research, with potential therapeutic implications. For example, interfering with B cell help provided by TFH cells has been shown to ameliorate autoimmune diseases in animal models and human patients.
In summary, TFH cells are defined by the expression of the transcription factor Bcl6, which plays a pivotal role in TFH cell differentiation and function through its regulatory actions on various genes and transcription factors. Further understanding of TFH biology, particularly the mechanisms underlying Bcl6-mediated gene regulation, may provide valuable insights into the treatment of biomedically relevant diseases.
Trump's Tax Returns: Can California Demand Disclosure?
You may want to see also

TFH cells play a critical role in protective immunity, helping B cells produce antibodies against foreign pathogens
T follicular helper (TFH) cells are a specialised subset of CD4+ T cells that play a critical role in protective immunity, helping B cells produce antibodies against foreign pathogens. TFH cells were first identified in the human tonsil and are located in secondary lymphoid organs (SLOs), including the tonsil, spleen, and lymph nodes. These organs contain separate T and B cell zones, and TFH cells are uniquely found in the B cell zone, where they interact closely with B cells.
TFH cells are essential for the formation of germinal centres (GCs), which are distinct structures that form within the B cell zones of SLOs during an active immune response. B cells within GCs, known as GC B cells, undergo rapid proliferation and antibody diversification, allowing the production of various antibodies with greater affinity for their targets. TFH cells direct this process by providing co-stimulation to B cells through the interaction of the co-stimulatory molecule CD40 on B cells with CD40-ligand (CD40L) on TFH cells, as well as by producing the cytokine IL-21, which drives B cell proliferation. The additional cytokine production by TFH cells helps determine the type of antibody produced.
TFH cells also play a critical role in mediating the selection and survival of B cells, which can differentiate into long-lived plasma cells capable of producing high-affinity antibodies against foreign antigens. Furthermore, TFH cells facilitate the negative selection of potentially autoimmune-causing mutated B cells in the germinal centre, helping to prevent the development of antibody-mediated autoimmune diseases.
The differentiation of TFH cells is a multi-step process that begins in the T cell zone of SLOs. Naive CD4+ T cells recognise protein peptides presented by dendritic cells through their T-cell receptor (TCR) and receive additional signals through co-stimulatory molecules and cytokine production, leading to their commitment to the TFH lineage. Developing TFH cells express the chemokine receptor CXCR5, which allows them to migrate towards the B cell zone. Fully differentiated TFH cells within GCs, known as GC TFH cells, express the highest levels of CXCR5, PD1, and ICOS, which are important for their function.
Understanding the biology of TFH cells and their role in protective immunity is of significant interest for improving vaccination strategies and treating autoimmune diseases. By harnessing emerging technologies and computational methods, researchers aim to enhance our knowledge of TFH cells and their potential as therapeutic targets.
The Key to a Good Sanger Sequencing Chromatogram
You may want to see also
Frequently asked questions
TFH cells, or Follicular Helper T cells, are a specialised subset of CD4+ T cells that play a significant role in the adaptive immune response. They are found in secondary lymphoid organs such as the tonsils, spleen, and lymph nodes.
TFH cells help B cells by promoting their development, proliferation, and production of affinity-matured antibodies. They also support the formation of germinal centres (GCs), which are necessary for B cell maturation and antibody production.
TFH cells are essential for the formation and maintenance of GCs. They express CD40L, which binds and stimulates the B cell surface receptor CD40, leading to B cell survival and differentiation. GCs are the sites where B cells differentiate into antibody-secreting plasma cells and memory B cells.
TFH cells play a critical role in protective immunity by helping B cells produce antibodies against foreign pathogens. They also facilitate negative selection of potentially autoimmune-causing mutated B cells in the germinal centre.
Individuals with autoimmunity often have an increased frequency of TFH cells in their blood. TFH cells are involved in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and primary Sjögren's syndrome (pSS). Elevated levels of TFH-like cells have been detected in patients with SLE and Sjögren's syndrome.