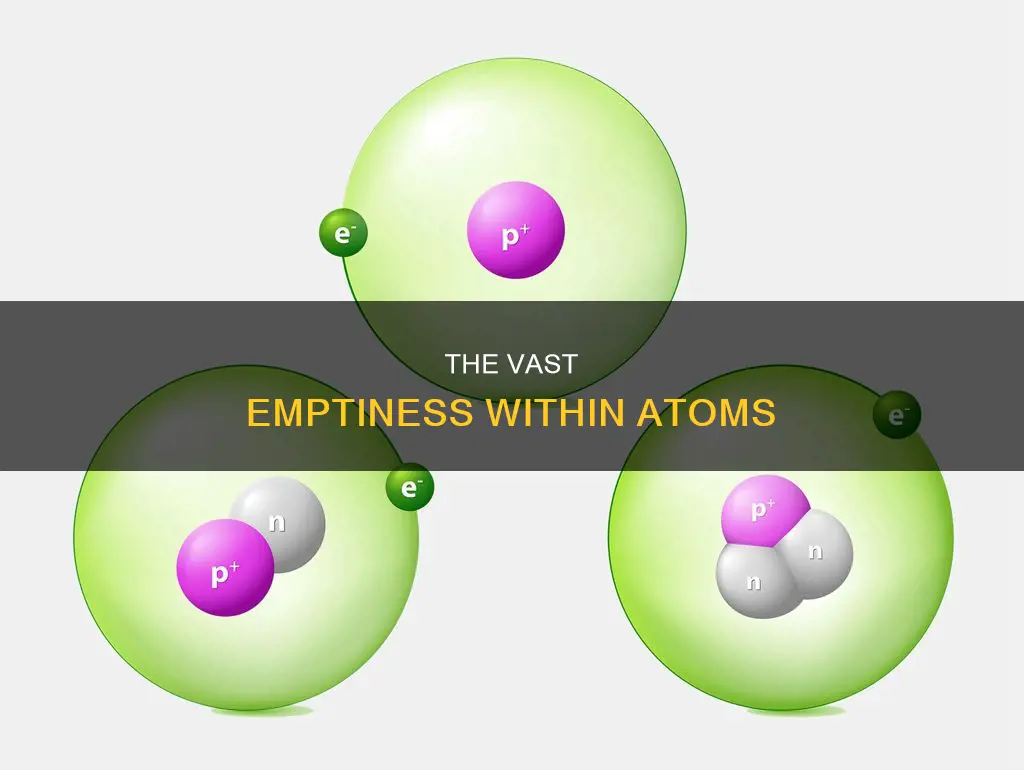
The atom is a fascinating structure, and its composition has been a subject of scientific inquiry for centuries. Atoms are composed of subatomic particles, including protons, neutrons, and electrons. While the nucleus of an atom, containing the protons and neutrons, accounts for most of its mass, it is the electrons that occupy almost all of its volume. This is because the nucleus is incredibly small compared to the atom as a whole, likening to a blueberry within a football stadium. Thus, the vast majority of an atom is empty space, with electrons whizzing around in this vast expanse.
| Characteristics | Values |
|---|---|
| Volume of an atom made up of | Protons, neutrons, electrons, and empty space |
| Nucleus made up of | Positively charged protons and uncharged neutrons |
| Diameter of an atom | 10^-10 m |
| Diameter of the nucleus | 10^-15 m |
| Relative size of the nucleus to an atom | 1:100,000 |
| Percentage of atomic mass occupied by the nucleus | 99.9994% |
| Percentage of atomic volume occupied by the nucleus | <0.0001% |
Explore related products
What You'll Learn

Protons
The mass of an atom is influenced by the number of protons and neutrons in its nucleus. Protons and neutrons have similar masses, with one proton approximately equal to the mass of one neutron. Therefore, the total number of protons and neutrons affects the overall mass of the atom. However, it is important to note that the mass of an atom is not solely determined by the number of protons, as different isotopes of the same element can have varying numbers of neutrons, resulting in slight mass differences.
The behavior of protons within an atom is governed by the strong nuclear force, which holds them together in the nucleus. This force is incredibly strong, allowing protons to overcome their mutual electrostatic repulsion since they all carry positive charges. The strong nuclear force also plays a role in nuclear reactions, where protons can be converted into neutrons or vice versa through processes like beta decay or electron capture.
In summary, protons are a fundamental component of atoms, contributing to their charge, mass, and unique elemental identity. They are concentrated in the tiny nucleus at the center of the atom, which makes up only a small fraction of the total volume but contains most of the mass due to the dense nature of protons and neutrons. Understanding protons and their behavior within atoms is essential for comprehending the broader field of chemistry and the behavior of matter at its most fundamental level.
George Washington's Role at the First Constitutional Congress
You may want to see also

Neutrons
While neutrons contribute significantly to an atom's mass, they do not make up a significant portion of its volume. In fact, the nucleus, which includes both protons and neutrons, occupies only a tiny fraction of an atom's total volume. The atom is mostly composed of empty space, with negatively charged electrons occupying almost its entire volume. This is because neutrons, along with protons, are much heavier than electrons, allowing them to be concentrated in a much smaller space.
The relative sizes of the atom and its nucleus can be compared to a football stadium and a blueberry, respectively. This helps to illustrate the vast amount of empty space within an atom, despite the mass being concentrated in the tiny nucleus.
Research supported by the DOE Office of Nuclear Physics in the Office of Science aims to further our understanding of nuclear matter. This includes studying how the structure of nuclei depends on the number of protons and neutrons they contain. By heating nuclei to the temperatures of the early universe, scientists can also gain insights into how they condensed out of the quark-gluon soup that existed at that time.
The Constitution's Main Writer: Uncovering the Founding Father's Legacy
You may want to see also

Electrons
Despite their small size and mass, electrons are highly significant in the context of atomic volume. This is because electrons occupy a much larger volume of space compared to the other components of an atom. The nucleus of an atom, which contains the protons and neutrons, is extremely small, with a diameter roughly 100,000 times smaller than the diameter of the atom itself. On the other hand, electrons are distributed in a cloud-like structure around the nucleus, taking up a substantial portion of the atom's volume.
The behavior of electrons is governed by the electromagnetic force, which results in an attractive force between the negatively charged electrons and the positively charged protons in the nucleus. This force prevents the electrons from escaping the pull of the nucleus and keeps them in orbit around it. However, it is important to note that electrons do not follow precise orbits like planets; instead, they exist as probability distributions, or electron clouds, that indicate the likelihood of finding an electron in a particular region of space.
The number of electrons in an atom is usually equal to the number of protons, resulting in a neutral atom. However, atoms can gain or lose electrons, becoming positively or negatively charged ions, respectively. This process of gaining or losing electrons is fundamental to the formation of chemical bonds and the behavior of elements in chemical reactions.
In summary, electrons are essential components of atoms, and their presence and behavior significantly influence the volume and properties of atoms. Their negative charge, distribution in electron clouds, and ability to be gained or lost contribute to the unique characteristics of each atom and their interactions with other atoms.
Understanding Constitutional Rules of Proceedings
You may want to see also
Explore related products

Empty space
An atom is primarily composed of empty space. The nucleus of an atom, which contains the majority of its mass, is incredibly small compared to the overall volume of the atom. If we imagine a hydrogen atom, with its nucleus the size of a football, the atom itself would be the size of a football stadium, with nothing but empty space between.
The diameter of an atom is approximately 10-10 m, while the diameter of the nucleus is about 10-15 m, or about 100,000 times smaller than the atom as a whole. This means that the nucleus occupies less than one ten-trillionth of the total volume of the atom.
Electrons, which are much less massive than protons and neutrons, occupy almost all of an atom's volume. They exist in a cloud of negative charge surrounding the nucleus, which contains the positively charged protons and neutral neutrons.
The concept of atoms being mostly empty space is a revelation of modern atomic theory, which has provided valuable insights into the inner structure of atoms. This theory has also helped us understand the relative sizes and distances involved, such as through the blueberry and football stadium analogy.
The fact that atoms are predominantly empty space helps explain certain phenomena, such as why cockroaches can survive nuclear explosions. The vast empty space within atoms allows for a great deal of flexibility and movement within their structure, even when subjected to extreme forces.
Technology's Influence on Constitutional Interpretation
You may want to see also

Nucleus size
The atom is composed of a small nucleus containing protons and neutrons, surrounded by a much larger volume of space containing electrons. Despite containing the majority of an atom's mass, the nucleus is extremely small, with a diameter of approximately 10−15 m, while the atom itself has a diameter of about 10−10 m. This means that the nucleus is roughly 100,000 times smaller than the atom as a whole. To put this into perspective, if the nucleus were the size of a blueberry, the atom would be as big as a football stadium.
The nucleus accounts for a significant portion of the atom's mass due to the presence of protons and neutrons, which are substantially heavier than electrons. In fact, the nucleus makes up over 99.9994% of the atom's total mass. However, it occupies a minuscule amount of the atomic volume, taking up less than one ten-trillionth of the space. This discrepancy in size and mass is because the electrons, which occupy most of the volume, are extremely lightweight in comparison.
The relative sizes of the nucleus and the atom, as well as their respective contributions to the total volume and mass, are crucial aspects of atomic structure. These characteristics have been discovered through the development of modern atomic theory, which has provided valuable insights into the inner workings of atoms.
While the nucleus is indeed very small compared to the atom as a whole, it is not the smallest component. Protons and neutrons, which make up the nucleus, are themselves composed of even smaller entities called quarks. Quarks are fundamental particles that come in several types, or "flavours," and they combine to form protons and neutrons through a force carried by particles called gluons.
The size of the nucleus can vary depending on the number of protons and neutrons it contains. This variation is an active area of research in nuclear physics, where scientists are working to understand how the structure of nuclei changes with different proton and neutron numbers. By studying the properties of nuclei, including their size, composition, and behaviour under extreme conditions, researchers can gain valuable insights into the fundamental nature of matter and the early universe.
Comparing the Charters: Germany and the US
You may want to see also
Frequently asked questions
Electrons occupy almost all of an atom's volume.
The diameter of an atom is approximately 10−10 m, while the diameter of the nucleus is about 10−15 m, making the nucleus about 100,000 times smaller than the atom.
The nucleus is composed of positively charged protons and uncharged neutrons.
Protons and neutrons are much heavier than electrons, so they account for most of the atom's mass.
The nucleus occupies less than one ten-trillionth of the atomic volume.