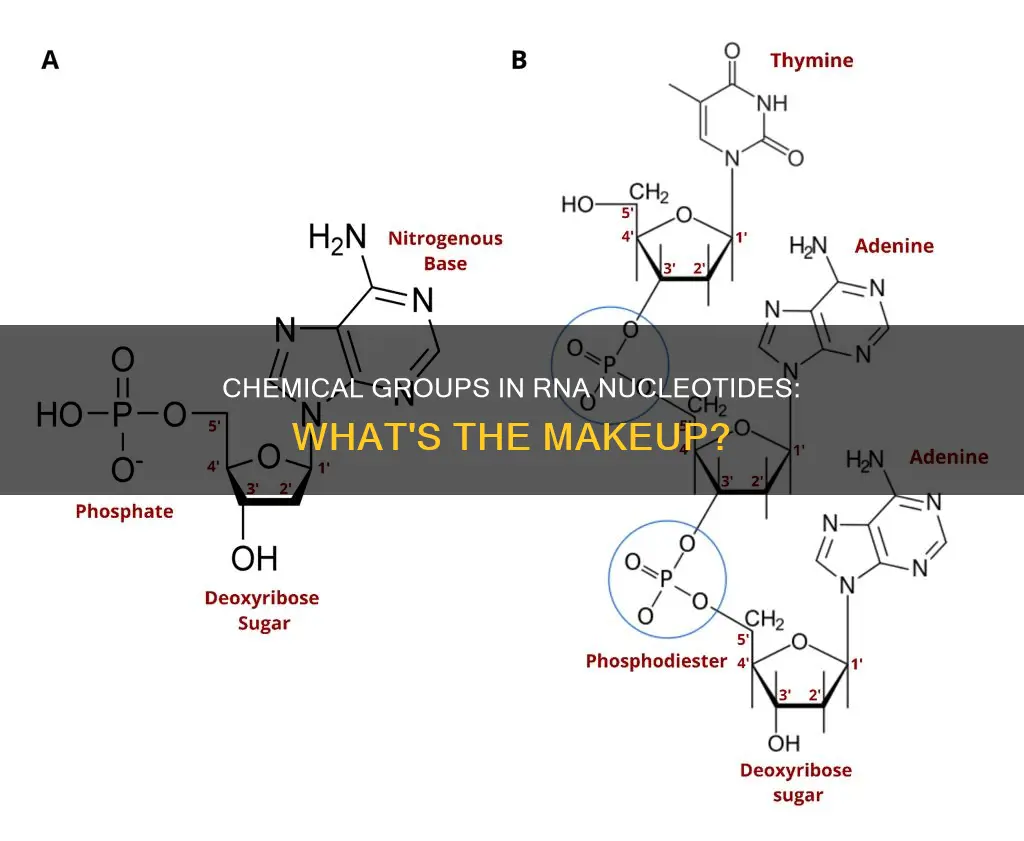
Nucleotides are composed of three subunit molecules: a five-carbon sugar (ribose), a phosphate group, and a nucleobase. The nucleotides found in RNA contain the nucleobases adenine, uracil, cytosine, and guanine. These nucleobases are attached to phosphate groups and ribose sugars. RNA differs from DNA in that it contains uracil instead of thymine and has a different sugar backbone.
| Characteristics | Values |
|---|---|
| Nitrogenous bases | Adenine, Cytosine, Guanine, Uracil |
| Sugar | Ribose |
| Phosphate group | 5'-phosphate group |
| Nucleobases | Purine, Pyrimidine |
| Nucleotide types | Adenine and Guanine (Purines), Cytosine and Uracil (Pyrimidines) |
Explore related products
What You'll Learn

Nitrogenous bases
Pyrimidines have a simple-ring structure and include cytosine, uracil, and thymine. Cytosine, chemically known as 2-oxy-4-aminopyrimidine, is present in both DNA and RNA. It has an amino group at the C4 position. Uracil, or 2,4-dioxypyrimidine, is found in RNA but not DNA. It is a demethylated form of thymine, with oxo groups substituted at C2 and C4. Thymine, or 5-methyluracil, is present in DNA but not RNA.
Purines, on the other hand, have a fused-ring skeletal structure. The major purine bases are adenine and guanine, which are found in RNA. Other purines include methyladenine, methylguanine, hypoxanthine, and xanthine. Purine biosynthesis involves the use of residues such as glycine, glutamine, aspartate, formyl tetrahydrofolate, and CO2.
In RNA, the nitrogenous bases of guanine, uracil, adenine, and cytosine, denoted by the letters G, U, A, and C, are used to convey genetic information that directs the synthesis of specific proteins. These bases form hydrogen bonds: cytosine pairs with guanine, adenine with uracil, and guanine with uracil.
Understanding the Quorum for Corporate Business Transactions
You may want to see also

Phosphate groups
The phosphate groups play a vital role in the structure and function of RNA. They contribute to the negative charge of RNA, making it a charged molecule (polyanion). This charge is due to the presence of a negative charge on each phosphate group. The phosphate groups also participate in forming the 5'-3' phosphodiester bonds between ribose sugars, linking the individual ribonucleotides together into a long chain.
In addition to their structural role, phosphate groups are involved in various cellular processes. They serve as a source of energy for the cell, providing chemical energy in the form of nucleoside triphosphates such as ATP (adenosine triphosphate) and GTP (guanosine triphosphate). These nucleoside triphosphates are essential for cellular functions like amino acid synthesis, protein synthesis, and cell membrane synthesis.
Moreover, phosphate groups are important in cellular signaling. They can modulate the activity of proteins and other signaling molecules. For example, cyclic nucleotides, which are signaling molecules, are formed by binding the phosphate group twice to the same sugar molecule, bridging the 5' and 3' hydroxyl groups. Some signaling nucleotides may also have multiple phosphate groups attached to different positions on the sugar molecule.
The presence of phosphate groups in RNA is not only important for the structure and function of the RNA molecule itself but also plays a vital role in maintaining the overall cellular processes and activities.
The Constitution's Influence on Senate and House Procedures
You may want to see also

Ribose sugars
Ribose is one of the key differences between RNA and DNA. While RNA contains ribose, the sugar in DNA is called deoxyribose. The "deoxy" prefix indicates that RNA has two hydroxyl (-OH) groups attached to its carbon backbone, while DNA has only one and has a lone hydrogen atom attached instead. This extra hydroxyl group in RNA contributes to its conformation and is useful in the process of converting genetic code into mRNAs that can be made into proteins. On the other hand, the deoxyribose sugar in DNA provides more stability to the molecule.
The ribose sugar in RNA is part of the nucleotide's sugar-phosphate backbone. This backbone is formed by covalently binding the nitrogenous bases to the ribose sugar, and then linking the phosphate group to the 5' carbon of the ribose. The phosphate groups have a negative charge each, making RNA a charged molecule (polyanion). The phosphate groups also play a crucial role in forming the phosphodiester bonds that connect individual nucleotides into a chain.
The ribose sugar in RNA is essential for the molecule's structure and function. It contributes to the flexibility of RNA, allowing it to adopt various conformations, including single-stranded and double-stranded structures. The ribose sugar also participates in base pairing through hydrogen bonding. In RNA, the purine bases adenine and guanine, and the pyrimidine base cytosine, can pair with uracil, another pyrimidine base. The hydrogen bonding between these bases contributes to the secondary structure of RNA.
Overall, ribose sugars are a fundamental component of RNA nucleotides, contributing to the molecule's structure, flexibility, and functionality in genetic information transfer and protein synthesis.
The Constitution: Protecting Us From Tyranny
You may want to see also
Explore related products

Purine and pyrimidine bases
Purines and pyrimidines are the two families of nitrogenous bases that make up nucleic acids. They are the building blocks of DNA and RNA. The number of rings in the nitrogenous base determines whether it is a purine or a pyrimidine. Purines have two rings in their nitrogenous base, while pyrimidines have one. Adenine and guanine are the two main types of purine. The other purine bases are caffeine, theobromine, uric acid, and isoguanine. Cytosine and uracil are pyrimidines.
Purines always bind with pyrimidines, which is known as complementary pairing. This means that the ratio of the two will always be constant within a DNA molecule. This phenomenon is known as Chargaff’s Rule, named after Irwin Chargaff, who first noticed it. In DNA, adenine pairs with thymine, and guanine pairs with cytosine. In RNA, thymine is replaced by uracil.
Purines and pyrimidines have similar functions within DNA and RNA molecules. They form the basic structure of nucleic acids, which are the genetic material of living organisms. The purines on one strand of DNA form hydrogen bonds with the corresponding pyrimidines on the opposite strand, and vice versa, to hold the two strands together. The number of hydrogen bonds between them is constant. Adenine pairs with thymine/uracil with two hydrogen bonds, while guanine pairs with cytosine with three hydrogen bonds.
Purines and pyrimidines are formed by two pathways: de novo and salvage. Besides forming nucleic acids, purines also form biomolecules such as ATP, NAD, GTP, cyclic AMP, and coenzyme A. Coenzyme A is involved in the citric acid cycle. ATP is the main energy source of cells. NAD is involved in redox reactions during glycolysis metabolism.
Policy Replacement: What Won't Make the Cut?
You may want to see also

Adenine, cytosine, guanine, and uracil
The nitrogenous bases of adenine, cytosine, guanine, and uracil play a crucial role in gene expression and protein synthesis. They form hydrogen bonds between specific pairs: adenine with uracil, cytosine with guanine, and guanine with uracil. These base pairs contribute to the secondary structure of RNA, while RNA folding creates its tertiary structure, resulting in a three-dimensional shape with helices and grooves.
In the context of RNA, adenine is one of the major purine bases, along with guanine. Purines have a double ring structure, with one ring similar to that of pyrimidines. Adenine is denoted by the letter "A" and plays a vital role in conveying genetic information and directing the synthesis of specific proteins.
Cytosine, represented by the letter "C," is a pyrimidine base present in both RNA and DNA. Its chemical formula is 2-oxy-4-aminopyrimidine, and it exhibits keto-enol tautomerism. Cytosine forms three hydrogen bonds with guanine, contributing to the stability and specificity of RNA molecules.
Guanine, denoted by the letter "G," is the other major purine base found in RNA. It has the chemical formula 2-amino-6-oxypurine and is involved in gene expression and protein synthesis. Along with adenine, it forms the nitrogenous bases of messenger RNA (mRNA), which carries genetic information and directs protein synthesis.
Uracil, represented by the letter "U," is one of the four chemical bases unique to RNA and is not found in DNA. It is a pyrimidine base structurally similar to thymine but with a different chemical formula, 2,4-dioxypyrimidine. Uracil pairs with adenine through two hydrogen bonds, contributing to the base pairing that is essential for RNA's secondary structure.
Bush's Constitution: Paper-Thin Respect for Democracy
You may want to see also
Frequently asked questions
Nucleotides found in RNA contain a five-carbon sugar called ribose, a phosphate group, and a nitrogenous base.
The four nitrogenous bases in RNA are adenine, uracil, cytosine, and guanine.
Unlike DNA, RNA contains uracil instead of thymine. RNA also contains ribose sugar instead of deoxyribose, which has an extra hydroxyl group on the second carbon.
The three primary types of RNA molecules are messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA).