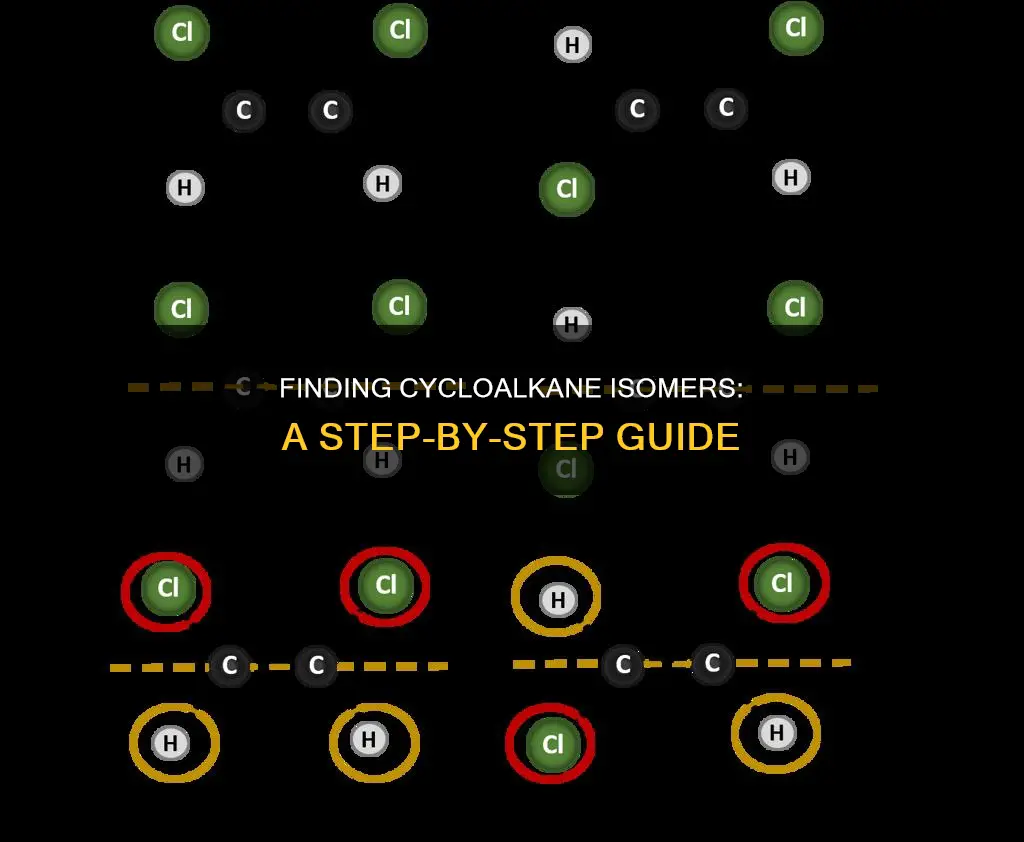
Constitutional isomers are compounds with the same molecular formula but different structural formulas. They are well-documented in organic chemistry, and their distinctions based on structural formulas showcase the principles of isomerism. For example, the molecular formula C4H8 represents a group of constitutional isomers, including alkenes and cycloalkanes. To identify constitutional isomers, one can start with a basic cyclooctane and make the ring smaller while lengthening a substituent. This process helps to understand the different configurations of the same molecule.
| Characteristics | Values |
|---|---|
| Definition | Constitutional isomers are compounds that have the same molecular formula but different connectivity. |
| Other Names | Cycloalkanes are also known as alkenes. |
| Molecular Formula | The molecular formula for cycloalkanes is C_nH_2n. |
| Structural Formula | Cycloalkanes have cyclic structures with single bonds between carbon atoms. |
| Examples | Examples of cycloalkanes include cyclobutane and methylcyclopropane. |
| Isomer Examples | The only constitutional isomer of cyclopropane is propene. |
| Identification | To identify constitutional isomers, look for compounds with the same molecular formula but different structural formulas. |
Explore related products
What You'll Learn
- Constitutional isomers are compounds with the same formula but different connectivity
- Cycloalkanes have the same general formula as alkenes
- Cycloalkanes have cyclic structures with single bonds between carbon atoms
- Cyclopropane is the simplest cycloalkane
- Monochlorination can result in constitutional isomers

Constitutional isomers are compounds with the same formula but different connectivity
Constitutional isomers are compounds with the same molecular formula but different structural formulas. In other words, they have the same number of atoms of each element but differ in the way these atoms are connected. For example, consider the case of cyclopropane (C3H6) and propene. Both compounds share the molecular formula C3H6, but they have different structures. Cyclopropane has a ring structure, while propene has a straight-chain structure with a double bond. Thus, they are constitutional isomers of each other.
Constitutional isomers are a type of isomer, which refers to molecules that share the same molecular formula but differ in other ways. There are several types of isomers, including constitutional isomers, stereoisomers, enantiomers, and diastereomers. Stereoisomers have the same connectivity between atoms but differ in their arrangement in space. Enantiomers are a type of stereoisomer that are non-superimposable mirror images of each other. Diastereomers are also stereoisomers but are not mirror images of each other.
Constitutional isomers are well-documented in organic chemistry, particularly in the context of alkenes and cycloalkanes. Alkenes are compounds that contain carbon-carbon double bonds, while cycloalkanes are compounds that have cyclic structures with single bonds between carbon atoms. For example, the molecular formula C4H8 can have constitutional isomers that are alkenes, such as but-1-ene and but-2-ene, or cycloalkanes, such as cyclobutane and methylcyclopropane.
The number of possible constitutional isomers increases with the number of carbon atoms. For example, while there is only one possible isomer for CH4 (methane), there are 355 possible isomers for dodecane (C12H26). When identifying constitutional isomers, it is important to consider both the molecular formula and the structural formula, as compounds with the same molecular formula can have different structures, resulting in different properties such as boiling point, melting point, and chemical reactivity.
To determine the constitutional isomers of a cycloalkane, one approach is to start with a basic cyclooctane structure and then make the ring smaller while lengthening a substituent. For instance, starting with C8H16, one can draw methylcycloheptane, ethylcyclohexane, and propylcyclopentane by following this approach. By reducing the size of the ring and adding to the substituent, one can identify various constitutional isomers with the same molecular formula but different connectivities.
Founding Fathers: Constitution Creation Story
You may want to see also

Cycloalkanes have the same general formula as alkenes
When identifying constitutional isomers of a cycloalkane, it is important to understand that constitutional isomers refer to compounds with the same molecular formula but different structural arrangements or connectivities. In other words, they have the same number of atoms of each element but are connected differently.
Cycloalkanes are a subclass of alkanes with one or more rings of carbon atoms, and they follow the general formula CnH2n, where 'n' represents the number of carbon atoms in the molecule. For example, cyclohexane (C6H12) has six carbon atoms bonded to each other in a ring structure, with each carbon atom bonded to two hydrogen atoms.
Alkenes, on the other hand, are hydrocarbons that contain a carbon-carbon double bond and have the general formula CnH2n, where 'n' is the number of carbon atoms. Despite having the same general formula as cycloalkanes, alkenes have a different molecular structure due to the presence of the double bond. For instance, 1-hexene (C6H12), an alkene, has six carbon atoms and twelve hydrogen atoms, just like cyclohexane, but its carbon atoms form a chain with a double bond between two of the carbons.
Constitutional isomers of cycloalkanes can be determined by considering the number of carbon atoms and their arrangement in the ring structure. For example, C8H16 can have constitutional isomers such as cyclooctane, methylcycloheptane, ethylcyclohexane, and propylcyclopentane. By following the pattern of reducing the ring size and lengthening the substituent, we ensure that the number of carbon and hydrogen atoms remains the same while changing the connectivity.
In summary, cycloalkanes and alkenes can have the same general formula, but they differ in their molecular structure. Cycloalkanes have carbon atoms arranged in rings, while alkenes have a carbon-carbon double bond. Constitutional isomers of cycloalkanes can be identified by modifying the ring structure while maintaining the same molecular formula.
Religious Holiday Displays: Public Property, Constitutional?
You may want to see also

Cycloalkanes have cyclic structures with single bonds between carbon atoms
When identifying constitutional isomers, it is important to understand that these are compounds with the same molecular formula but different structural arrangements of constituent atoms. In the context of cycloalkanes, this means that the isomers will have the same molecular formula but will differ in their carbon atom arrangements.
Cycloalkanes are a group of hydrocarbons with a cyclic structure, meaning their carbon atoms form rings. The simplest cycloalkane is cyclopropane (C3H6), which has a triangular structure with each carbon atom connected to two hydrogen atoms. Its only constitutional isomer is propene, a straight-chain structure with a double bond. This is an example of an alkene isomer, which is common among cycloalkanes.
For the molecular formula C4H8, there are multiple constitutional isomers, including both alkenes and cycloalkanes. Examples of alkenes are but-1-ene and but-2-ene, while examples of cycloalkanes are cyclobutane and methylcyclopropane. These isomers differ in the arrangement of their carbon atoms, with alkenes having double bonds between carbon atoms, and cycloalkanes forming rings.
The number of possible isomers increases with the number of carbon atoms. For example, dodecane (C12H26) has 355 possible isomers. When identifying constitutional isomers, it is important to consider the different structural arrangements of carbon atoms and the presence of double or triple bonds.
To find constitutional isomers of cycloalkanes, one approach is to start with a basic structure and modify it. For instance, starting with cyclooctane (C8H16), you can make the ring smaller and lengthen a substituent. This results in isomers such as methylcycloheptane, ethylcyclohexane, and propylcyclopentane. By systematically altering the ring size and substituents, you can identify various constitutional isomers of cycloalkanes with different carbon atom arrangements while maintaining the same molecular formula.
Sex and Marriage: What God Sees
You may want to see also
Explore related products

Cyclopropane is the simplest cycloalkane
Cyclopropane, with the molecular formula (CH2)3, is the simplest cycloalkane. It consists of three methylene groups (CH2) linked together to form a triangular ring. The small size of the ring creates substantial ring strain in the structure, which is calculated to be around 120 kJ mol−1. This strain is due to the deviation from the ideal tetrahedral bond angle of 109° 28', with cyclopropane requiring a bond angle of 60° between its carbon-carbon covalent bonds. The unusual structure of cyclopropane has led to theories invoking σ-aromaticity to explain its relatively low strain compared to cyclobutane.
Cyclopropane has a variety of unique properties due to its structure. For example, it has weaker carbon-carbon bonds than ordinary carbon-carbon bonds, but stronger carbon-hydrogen bonds. It also has torsional strain due to the eclipsed conformation of its hydrogen atoms. Despite its strain energy, cyclopropane does not exhibit explosive behaviour substantially different from other alkanes.
The preparation of cyclopropane rings is referred to as cyclopropanation. While cyclopropane itself is mainly of theoretical interest, many of its derivatives, also known as cyclopropanes, are of commercial or biological significance. Cyclopropane was discovered in 1881 by August Freund, who also proposed its correct structure. It was first produced via a Wurtz coupling reaction, in which 1,3-dibromopropane was cyclized using sodium. The yield of this reaction was improved by using zinc as the dehalogenating agent and sodium iodide as a catalyst.
Cyclopropane has been used in various applications, such as an anaesthetic agent in clinical settings from the 1930s to the 1980s. Its anaesthetic properties were discovered by Henderson and Lucas in 1929, and it was introduced into clinical use by the American anaesthetist Ralph Waters. However, due to its high flammability and risk of fire and explosions, it has been superseded by other agents in modern anaesthetic practice.
The Constitution: Military and Federal Government Explained
You may want to see also

Monochlorination can result in constitutional isomers
Monochlorination is an organic reaction in which a loosely held hydrogen atom is replaced by a chlorine atom, initiated by light energy. The resulting compounds are known as constitutional isomers. These isomers have the same molecular formula but different structural arrangements of constituent elements.
For example, the monochlorination of 2,2-dimethylhexane results in five different types of hydrogen atoms, yielding five constitutional isomers. Similarly, the monochlorination of n-heptane results in four different types of hydrogen atoms, leading to four constitutional isomers.
The number of possible constitutional isomers increases with the complexity of the molecule. For instance, in the monochlorination of propane, two constitutional isomers are possible: 1-chloropropane and 2-chloropropane. However, when considering the monochlorination of pentane, there are three constitutional isomers: 1-chloropentane, 2-chloropentane, and 3-chloropentane.
It is worth noting that the yield of constitutional isomers from monochlorination may not always be predictable. For example, in the chlorination of propane, one might expect a higher yield of 1-chloropropane due to the larger number of methyl hydrogens. However, experimental results show a higher yield of 2-chloropropane, indicating that other factors influence the outcome of monochlorination reactions.
In summary, monochlorination can indeed result in the formation of constitutional isomers. The number and types of isomers depend on the specific molecule undergoing monochlorination, and the process involves the substitution of hydrogen atoms with chlorine atoms at different positions within the molecule.
The Constitution and Political Parties: System Established?
You may want to see also
Frequently asked questions
Constitutional isomers are compounds that have the same molecular formula but different structural formulas.
To identify constitutional isomers, you need to look for compounds with the same molecular formula as the given cycloalkane but different structural arrangements of atoms.
The constitutional isomers of C3H6 (cyclopropane) include propene, which has a double bond and a straight-chain structure. For C4H8, the constitutional isomers include but-1-ene, but-2-ene, and 2-methylprop-1-ene.
To draw constitutional isomers of cycloalkanes, start with a basic structure and then modify it. For example, you can start with cyclooctane and make the ring smaller while lengthening a substituent. This will result in isomers such as methylcycloheptane, ethylcyclohexane, and propylcyclopentane.











































