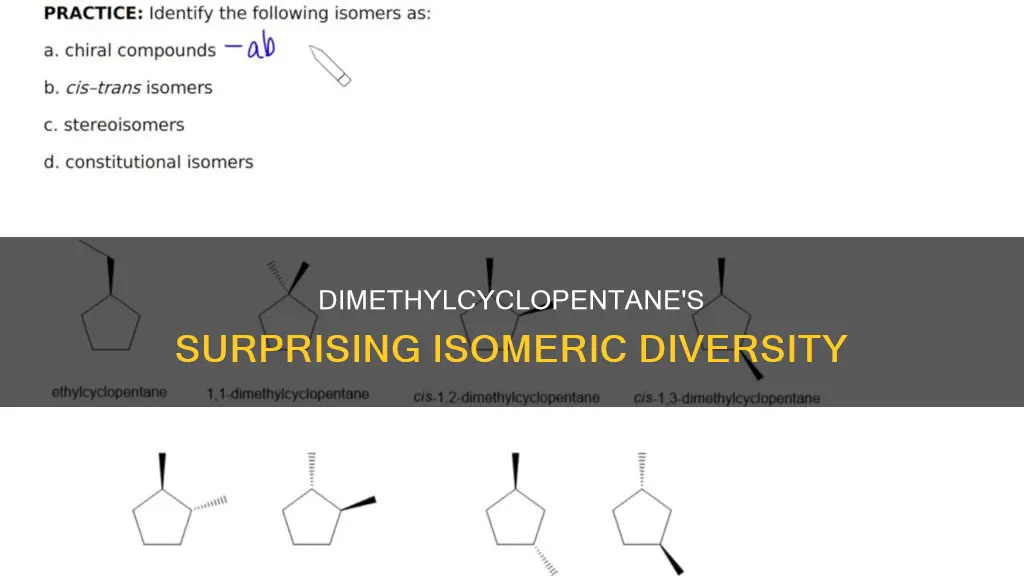
Dimethylcyclopentane is an organic compound with the formula C7H14. It has various isomers, including 1,1-, 1,2-, and 1,3-dimethyl cyclopentanes, which can further generate stereoisomeric and diastereomeric forms. The number of stereoisomers depends on the number of chiral centers in the molecule, with the formula for the maximum number given as X = 2^n, where n is the number of stereocenters. For example, 2,3-dimethylcyclobutane has three isomers: one cis isomer and two enantiomeric trans isomers. Similarly, 1,3-dimethylcyclobutane has two stereoisomers that are configurational isomers and exist as two enantiomers.
Explore related products
What You'll Learn

Dimethylcyclopentane can generate stereoisomeric forms
Dimethylcyclopentane, C7H14, has three positional isomers: 1,1-dimethylcyclopentane, 1,2-dimethylcyclopentane, and 1,3-dimethylcyclopentane. These isomers can generate stereoisomeric and diastereomeric forms.
Stereoisomers are molecules that have the same molecular formula and sequence of bonded atoms but differ in the spatial arrangement of their atoms in three dimensions. This can be due to the way atoms are oriented around a double bond or how the atoms are arranged around a ring structure, as in the case of dimethylcyclopentane.
Dimethylcyclopentane has seven isomers, including stereoisomers. This means that there are seven unique arrangements of the atoms in dimethylcyclopentane that result in distinct molecules, some of which are stereoisomers of each other.
Stereoisomers can be further classified as enantiomers or diastereomers. Enantiomers are stereoisomers that are mirror images of each other and are not superimposable. Diastereomers, on the other hand, are stereoisomers that are not mirror images and have different arrangements of atoms in space.
The specific stereoisomeric forms of dimethylcyclopentane and their classifications would depend on the specific structure and arrangement of atoms in each isomer. These stereoisomers can be determined through experimental methods, such as nuclear magnetic resonance (NMR) spectroscopy, or by applying the rules of stereochemistry to the structural formulas of the isomers.
Seven Years of US Citizenship: My Cityzen Story
You may want to see also

The number of stereoisomers is 2n, where n is the number of chiral centres
The number of stereoisomers of a molecule is often represented by the formula 2^n, where n is the number of chiral centres. This formula indicates that the number of stereoisomers increases exponentially with the number of chiral centres in a molecule. For example, a molecule with three chiral centres would have 2^3 = 8 possible stereoisomers. However, it's important to note that this formula doesn't always hold true, as the actual number of stereoisomers depends on the specific structure and properties of the molecule.
In the case of dimethylcyclopentane, the molecule can have different isomers, including structural isomers and stereoisomers. Structural isomers occur when the atoms or functional groups are connected in different ways, resulting in distinct structures. On the other hand, stereoisomers have the same molecular formula and connectivity of atoms but differ in the spatial arrangement of the atoms in three-dimensional space.
Dimethylcyclopentane, with the molecular formula C7H14, can have various isomers due to the presence of two methyl groups that can be positioned at different places around the cyclopentane ring. These isomers include 1,1-dimethylcyclopentane, 1,2-dimethylcyclopentane, and 1,3-dimethylcyclopentane. Each of these positional isomers can further exhibit stereoisomerism, leading to the formation of stereoisomeric pairs.
The number of stereoisomers for a molecule with n chiral centres is often assumed to be 2^n. This formula arises because each chiral centre can exist in two possible configurations, typically referred to as R (rectus) and S (sinister). When a molecule has multiple chiral centres, the substitution of a particular group at each centre can result in different stereoisomers. For example, if a molecule has two chiral centres, it can form four stereoisomers: RR, RS, SR, and SS.
However, it's important to note that the actual number of stereoisomers observed may be less than 2^n due to factors such as symmetry or equivalent diastereoisomers. In some cases, certain diastereoisomers may be identical in structure, reducing the overall count of unique stereoisomers. This deviation from the 2^n rule occurs when the molecule possesses a plane of symmetry or when the stereoisomers are superimposable, resulting in fewer observable stereoisomers than theoretically predicted.
Exploring the Constitution's Reach in New Territories
You may want to see also

Cis- and trans-1,2-dimethylcyclopropane are stereoisomers
Dimethylcyclopentane isomers exist as both constitutional and stereoisomers. Constitutional isomers are molecules that share the same molecular formula but differ in atom connectivity. Stereoisomers, on the other hand, have the same molecular formula and atom connectivity, but their spatial orientation differs.
The term "cis" means "on this side" in Latin, indicating that both substituents are on the same side or face of the ring. In the cis isomer of 1,2-dimethylcyclopropane, both methyl groups are either on the upper face or the lower face of the ring. They occupy the same spatial region and are considered cis to each other.
On the other hand, the term "trans" means "across" in Latin, indicating that the substituents are on opposite sides or faces of the ring. In the trans isomer of 1,2-dimethylcyclopropane, one methyl group is on the upper face of the ring, while the other is on the lower face, making them trans to each other.
The cis and trans isomers of 1,2-dimethylcyclopropane have distinct properties due to their different spatial arrangements. Notably, the cis isomer is achiral, while the trans isomer exists as a pair of enantiomers, resulting in three stereoisomers of 1,2-dimethylcyclopropane overall.
Working Full-Time in Minnesota: Weekly Hour Requirements
You may want to see also
Explore related products

Dimethylcyclopropane's cis compound is achiral
Dimethylcyclopropanes have two isomers: 1,1-dimethylcyclopropane and 1,2-dimethylcyclopropane. The cis compound of 1,2-dimethylcyclopropane is achiral. This is because it consists of two enantiomeric conformations that interconvert rapidly via ring flipping at normal temperatures. This is similar to amine inversion, where the rapid inversion of configuration leads to the interconversion of the two enantiomeric forms.
The concept of chirality is central to understanding why the cis compound of 1,2-dimethylcyclopropane is achiral. Chirality refers to the geometric property of an object or spatial arrangement of atoms, where it is non-superposable on its mirror image. In other words, if an object or arrangement of atoms can be superimposed on its mirror image, it is described as being achiral.
The trans conformers of 1,2-dimethylcyclopropane, specifically (1R,2R) and (1S,2S), are both chiral and are enantiomers of each other. On the other hand, the cis conformer, (1R,2S), is achiral. This is because the cis conformer does not possess a plane of symmetry, but rather consists of two enantiomeric conformations that can rapidly interconvert.
The achirality of the cis compound of 1,2-dimethylcyclopropane classifies it as a meso-compound. According to the IUPAC Gold Book, a meso-compound is an achiral member of a set of diastereoisomers that also includes one or more chiral members. In the case of 1,2-dimethylcyclopropane, the cis compound is the achiral member, while the trans compound is the chiral member.
Client Payment Issues: Confidentiality Breach?
You may want to see also

Trans compound exists as a pair of enantiomers
Dimethylcyclopentane, C7H14, has 1,1, 1,2, and 1,3-dimethyl cyclopentanes, which can generate stereoisomeric and diastereomeric forms.
Now, onto the concept of a trans compound existing as a pair of enantiomers. Enantiomers are molecules that are mirror images of each other but are not superimposable. They are stereoisomers, which means they have identical physical properties, except for optical rotation. For example, in a chiral silicon compound, the (+) enantiomer rotates plane-polarized light clockwise, while the (-) enantiomer rotates plane-polarized light in the opposite direction. These enantiomers have the same molecular weight, density, melting point, boiling point, and odour.
Enantiomers can be further distinguished by their R/S designations, which are determined by the configurations of their chiral centers. However, it is important to note that molecules with chiral centers and a plane of symmetry may appear to be enantiomers but are actually the same, known as meso compounds.
Trans compounds, on the other hand, refer to a specific type of isomerism called geometric isomerism or cis-trans isomerism. Unlike simple tetrahedral compounds, trans isomers have two groups placed opposite each other on a tetrahedron. While trans isomers are not the same as enantiomers, it is possible for a molecule to have both types of isomers. For example, a molecule with one chiral center can exist as both a pair of enantiomers and a trans pair of enantiomers.
In summary, while trans compounds typically refer to geometric isomers, it is possible for a trans compound to also exist as a pair of enantiomers if it meets the criteria for both types of isomerism.
Pension Payouts: Capital Gains Tax Implications?
You may want to see also
Frequently asked questions
There are three structural isomers of dimethylcyclopentane.
There are four possible stereoisomers of dimethylcyclopentane.
Yes, dimethylcyclopentane can also generate stereoisomeric and diastereomeric forms.
Yes, 1,2-dimethylcyclopropane has three stereoisomers, and 2,3-dimethylcyclobutane has two enantiomeric trans isomers and one cis isomer.































