Alkanes are hydrocarbons that consist solely of single bonds between carbon atoms and have the general molecular formula C_nH_(2n+2). Pentane, or C5H12, is the simplest alkane with three constitutional isomers: pentane, isopentane, and neopentane. These compounds have the same molecular formula but differ in their structural formulas and atom arrangement. This is known as constitutional isomerism, where isomers have the same atoms but differ in their connectivity.
| Characteristics | Values |
|---|---|
| Number of carbon atoms | 5 |
| Number of hydrogen atoms | 12 |
| Number of isomers | 3 |
| Types of isomers | Structural, positional, geometrical, optical |
| Examples of isomers | Pentane, Isopentane, Neopentane |
| Other names | Methylbutane, Dimethylpropane |
Explore related products
What You'll Learn
- Pentane, isopentane, and neopentane are three valid isomers
- Structural isomers have the same formula but different connectivity
- Constitutional isomers have the same carbon atoms but differ in hydrogen atoms
- Alkanes are hydrocarbons with single bonds between carbon atoms
- Alkanes are also known as aliphatic compounds, derived from the Greek word for fat

Pentane, isopentane, and neopentane are three valid isomers
The molecular formula C5H12 refers to a group of isomers known as pentanes, which are alkanes with five carbon atoms. There are three 5-carbon alkane constitutional isomers with the molecular formula C5H12: pentane, isopentane, and neopentane. These compounds are isomers, meaning they share the same molecular formula but have different structural formulas and connectivity of atoms.
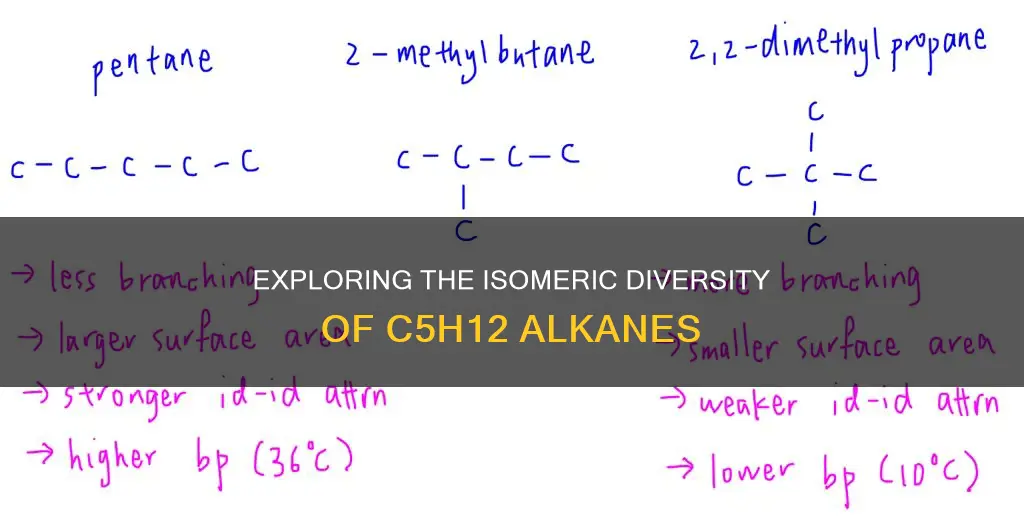
Pentane, or n-pentane, is the straight-chain isomer with the chemical formula C5H12. It is the simplest alkane and the fifth member of the alkane family. N-pentane has a boiling point of 36.1°C and is the preferred isomer for blowing agents in expanded polystyrene and polyethylene manufacturing. It is also used as a solvent in column chromatography.
Isopentane, also known as 2-methylbutane, is one of the branched-chain isomers of pentane. It has a chemical formula of C5H12 and exists as a colorless, transparent, volatile liquid with a pleasant aroma and a boiling point of 28°C. Isopentane is often used as a co-blowing agent alongside n-pentane in rigid polyurethane foam production.
Neopentane, also known as 2,2-dimethylpropane, is the other branched-chain isomer of pentane with the formula C5H12. It has the lowest boiling point among the three isomers at 10°C and exists as a gas at room temperature. Neopentane is a toxic chemical product obtained through the catalytic cracking and thermal decomposition of natural gas or petroleum.
While the physical properties of n-pentane, isopentane, and neopentane are similar, their chemical properties differ due to their distinct structures. The more branched isomers, isopentane and neopentane, exhibit higher stability, higher vapor pressures, and higher flash points compared to n-pentane. The three pentane isomers are often sold as a mixture, taking advantage of their different boiling and melting points.
The Power Players: Who Holds the Most Influence in America?
You may want to see also

Structural isomers have the same formula but different connectivity
There are three 5-carbon alkane constitutional isomers with the molecular formula C5H12. These are pentane, isopentane, and neopentane. These isomers have the same number of carbon and hydrogen atoms but differ in their structural formulas.
Now, let's delve into the concept of structural isomers. Structural isomers, also known as constitutional isomers, are molecules that have the same molecular formula but differ in the arrangement of their atoms. In other words, they have the same parts but are connected or structured differently. For example, consider the molecular formula C3H7Br. There are two structural isomers with this formula. In one isomer, the bromine atom is at the end of the chain, while in the other, it is attached in the middle. You cannot simply twist or rotate one molecule to transform it into the other; instead, you would need to break and re-attach the bromine atom and move a hydrogen atom to create the second isomer.
Another example of structural isomers is propanal (an aldehyde) and propanone (a ketone), which share the molecular formula C3H6O. These isomers belong to different families of compounds, known as homologous series, as they contain different functional groups. Structural isomers can also be observed in butane (C4H10). Butane can exist as a straight-chain structure or a branched chain, resulting in two distinct isomers. It is important to distinguish between true structural isomers and "false" isomers, which are simply twisted or rotated versions of the original molecule.
The concept of structural isomerism extends beyond organic molecules. For instance, in alcohols, there can be two structural isomers with a four-carbon chain, such as C4H9OH. Additionally, position isomers can occur on benzene rings, as demonstrated by the molecular formula C7H7Cl.
In summary, structural isomers, as exemplified by the C5H12 alkane isomers, exhibit the same molecular formula but differ in the connectivity or arrangement of their atoms. This results in distinct molecular structures, such as the linear and branched forms of 5-carbon alkanes. Understanding structural isomerism is not only intellectually stimulating but also has practical applications in various fields, including organic chemistry and materials science.
Debate, Compromise, and the Constitution's Evolution
You may want to see also

Constitutional isomers have the same carbon atoms but differ in hydrogen atoms
In chemistry, isomers are two or more molecules that share the same molecular formula but differ in structural formulas. One type of isomer is a constitutional isomer, also known as a structural isomer. Constitutional isomers have the same molecular formula but differ in the arrangement of atoms in their structures, resulting in different connectivity between atoms. This means that constitutional isomers have the same number of carbon atoms but can differ in the number of hydrogen atoms. For example, ethanol (CH3CH2OH) and dimethyl ether (CH3OCH3) both have the molecular formula C2H6O but differ in their connectivity. Ethanol's hydroxyl group (-OH) leads to hydrogen bonding, giving it unique properties such as a higher boiling point compared to dimethyl ether, which lacks this connectivity.
The molecular formula for alkanes is C_nH_(2n+2), where n is the number of carbon atoms. For 5-carbon alkane isomers, there are three valid constitutional isomers: pentane, isopentane, and neopentane. These isomers have the same molecular formula, C5H12, but differ in their structural formulas. Pentane is a straight chain of five carbon atoms, while isopentane has four carbon atoms in one chain with a methyl branch, and neopentane has a central carbon bonded to four other carbons, forming a branched structure.
The process of drawing out structural isomers for compounds is a great practice for understanding isomerism. For example, to find the structural isomers of C5H12, you can start by drawing out a stick structure of pentane with five carbons attached to one another with four carbon-carbon bonds. Then, you can move the carbons around to see how many different structural isomers you can create. This approach helps to visualize the different arrangements of atoms that can lead to the formation of constitutional isomers.
It is important to note that not all sets of molecular formulas represent valid constitutional isomers. For a set of formulas to represent constitutional isomers, each formula must have the same number of carbon atoms. Additionally, the presence of double or triple bonds or cyclic structures can affect the number of hydrogens present, so these considerations should also be taken into account when identifying constitutional isomers.
Private Property Protests: A Constitutional Right?
You may want to see also
Explore related products

Alkanes are hydrocarbons with single bonds between carbon atoms
Alkanes are a series of compounds that contain carbon and hydrogen atoms with single covalent bonds, also known as saturated hydrocarbons. They are the simplest family of hydrocarbons, consisting only of carbon and hydrogen. Each carbon atom forms four bonds, and each hydrogen atom forms one bond. The general molecular formula for straight and branched-chain alkanes is CnH2n+2, and that of cyclic alkanes is CnH2n. The smallest cycloalkane is cyclopropane, and cyclohexane is another example, with a ring structure known as the "chair" form.
Alkanes can be linear, branched, or cyclic in structure. The simplest alkane is methane (CH4), followed by ethane (C2H6), propane (C3H8), and butane (C4H10). Butane, for example, has an isomer called 2-methylpropane, demonstrating structural isomerism. All alkanes with 4 or more carbon atoms show structural isomerism, meaning there are two or more different structural formulas for each molecular formula.
Constitutional isomers, or structural isomers, of alkanes have the same molecular formula but different structural formulas. For example, C5H12 has three 5-carbon alkane constitutional isomers: pentane, isopentane, and neopentane. These isomers differ in their structural arrangements, with pentane being a straight chain of five carbon atoms, isopentane having four carbon atoms in one chain with a methyl branch, and neopentane forming a branched structure with a central carbon bonded to four other carbons.
The presence of a substituent, such as a halogen, in an alkane molecule can result in the conversion of one carbon-hydrogen bond to a carbon-substituent bond. For instance, when methane reacts with chlorine, it forms chloromethane, which consists of a CH3 group bonded to a chlorine atom. When an alkane loses a hydrogen atom from one bond, it becomes an alkyl group, often denoted by the letter R.
Alkanes are important in organic chemistry as they serve as the basis for naming many organic compounds. Additionally, they can be used to form substituent groups, such as the vinyl group (H2C=CH–) and the allyl group (H2C=CH–CH2–), which are commonly found in organic molecules.
The Constitution's Guard Against Tyranny: A Historical Analysis
You may want to see also

Alkanes are also known as aliphatic compounds, derived from the Greek word for fat
Alkanes, also known as aliphatic compounds, are derived from the Greek word 'aleiphar', meaning fat or oil. They are a type of hydrocarbon, which are compounds composed solely of carbon and hydrogen. Aliphatic compounds are further classified into two types: saturated and unsaturated. Saturated aliphatic compounds, like hexane, have all single C-C bonds, whereas unsaturated aliphatic compounds, like hexene and hexyne, have double or triple bonds.
Alkanes are a type of saturated aliphatic compound, where all the carbon atoms are connected by single bonds. The general formula for an alkane molecule is given by the equation CnH2n+2, where 'n' represents the number of carbon atoms in the molecule. For example, an alkane with five carbon atoms, or pentane, would have the molecular formula C5H12.
Alkanes with the same molecular formula but different structural formulas are known as structural isomers. For instance, pentane (a straight-chain alkane), isopentane (a branched-chain alkane), and neopentane (a unique branched-chain structure) are all structural isomers of the alkane C5H12. These isomers have different properties, including distinct boiling points.
The existence of isomers highlights the fascinating aspect of organic chemistry's three-dimensional nature. The shape and arrangement of atoms in a molecule can significantly impact its properties. Drawing out structural isomers is an excellent method for visualizing these variations and understanding the underlying principles of molecular connectivity and geometry.
In summary, alkanes, also known as aliphatic compounds, are a type of saturated hydrocarbon with only single bonds between carbon atoms. The name "aliphatic" originates from the Greek word for fat or oil. Alkanes can exhibit isomerism, where different structural arrangements of atoms result in distinct molecular properties, as exemplified by the isomers of C5H12.
The Constitution: A Long Road to Completion
You may want to see also
Frequently asked questions
There are three 5-carbon alkane constitutional isomers with the formula C5H12: pentane, isopentane, and neopentane.
Isomers are compounds with the same molecular formula but different structural formulas. They have the same numbers and kinds of atoms but differ in the way the atoms are arranged.
Constitutional isomers may have different carbon skeletons, different functional groups, or different locations of a functional group along the chain. They are always different compounds with different properties but with the same formula.
Alkanes are hydrocarbons that consist solely of single bonds between carbon atoms and have the general formula CnH2n+2, where n is an integer. They are often described as saturated hydrocarbons.


































