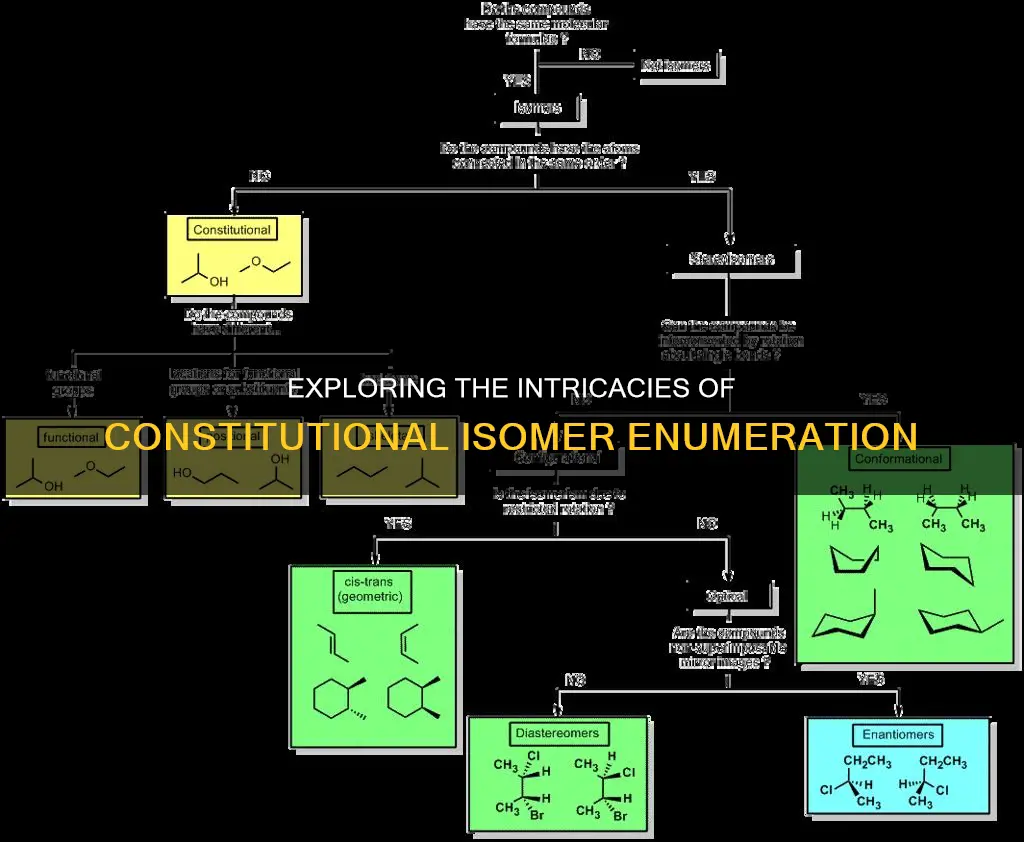
Constitutional isomers are organic compounds that have the same molecular formula but differ in their structural connectivity. They are identified by counting the number of carbon atoms and the degree of unsaturation (Hydrogen Deficiency Index or HDI). While there is no direct formula to calculate the number of constitutional isomers, the maximum number of stereoisomers can be calculated using the formula Nmax = 2^(n+m), where 'n' is the number of stereocentres and 'm' is the number of stereogenic double bonds.
| Characteristics | Values |
|---|---|
| Definition | Isomers are organic compounds that have the same molecular formula but have a different 3D arrangement of atoms. |
| Types | Constitutional isomers are the isomers that only differ in how they are structurally connected. |
| Formula | There is no direct formula to calculate the number of constitutional isomers. However, the maximum number of configurational stereoisomers (not constitutional isomers) is given by the formula \(N_\text{max}=2^{\left(n+m\right)}\), where \(n\) is the number of stereocentres (R or S) and \(m\) is the number of stereogenic double bonds (E or Z). |
| Identification | To identify constitutional isomers, create a molecular formula for the compound and count the number of carbons and heteroatoms. Compare the degree of unsaturation (Hydrogen Deficiency Index). |
Explore related products
What You'll Learn

Counting the number of carbons and heteroatoms
When dealing with organic compounds, carbon counts are particularly significant. The number of possible structural isomers increases exponentially with the number of carbons, making it challenging to enumerate or identify them manually. For instance, at 10 carbons, there are 75 isomers and 136 stereoisomers, and this complexity only grows with larger molecules. Therefore, software assistance is often required for accurate calculations.
To identify constitutional isomers, it is essential to create a molecular formula for the compound. This involves counting the number of carbons, hydrogens, and other elements present. For example, consider a compound with 7 carbon atoms, 14 hydrogens, one chlorine atom, and one fluorine atom. The molecular formula for this compound would be C7H14FCl.
The Index of Hydrogen Deficiency (IHD) or the Hydrogen Deficiency Index is another valuable tool for determining the number of constitutional isomers. The IHD helps predict the presence of double bonds and rings in a molecule. It is calculated by multiplying the number of carbon atoms by 2, adding the number of nitrogen atoms, adding 2, and then subtracting the number of hydrogen and halogen atoms. A higher IHD indicates more double bonds or rings in the molecule.
While there is no universal formula for determining the exact number of constitutional isomers, certain patterns and techniques can assist in the process. One approach is to start with the longest carbon chain or ring and gradually move to shorter ones, adding substituents, double bonds, and hydrogens accordingly. Additionally, the presence of stereogenic centres, such as chiral carbon atoms, can give rise to configurational isomers, further increasing the complexity of the molecule.
Marbury v. Madison: Judicial Review and Constitutional Violation
You may want to see also

Comparing the degree of unsaturation
The degree of unsaturation is a crucial concept in chemistry, particularly in the context of organic molecules and alkenes. It provides valuable insights into the molecular structure of a compound, including the presence of double bonds, triple bonds, and rings.
Calculating the degree of unsaturation involves determining the number of double bonds, triple bonds, and rings in a molecule. This calculation is based on the number of hydrogen atoms present in the molecule compared to the maximum number of hydrogen atoms each carbon atom can hold. For a molecule with 'n' carbon atoms, the maximum number of hydrogen atoms is represented by the formula 2n+2. Each double bond or ring in the molecule reduces the hydrogen count by two. Therefore, every ring or double bond is considered a "degree of unsaturation."
For example, let's consider the molecule benzene, which has the molecular formula C6H6. It has one ring and three double bonds, resulting in a total of four degrees of unsaturation. By doubling the degrees of unsaturation (4 x 2 = 8), we can determine the maximum number of hydrogens for a C6 compound, which is 14. The difference between the maximum possible hydrogens (14) and the actual number of hydrogens (8) further reinforces the presence of four degrees of unsaturation in benzene.
The concept of the degree of unsaturation is closely related to the index of hydrogen deficiency (IHD). The IHD formula helps calculate the number of double bonds and rings present in a molecule. It is a valuable tool for quickly determining the structure of an unknown compound with a known molecular formula. When calculating the degree of unsaturation, oxygen and sulfur atoms are typically ignored since they do not affect the saturation of a compound. On the other hand, nitrogen atoms are considered, and an additional hydrogen atom is added to achieve saturation when nitrogen is present.
In summary, comparing the degree of unsaturation involves analyzing the number of double bonds, triple bonds, and rings in a molecule. This comparison is facilitated by calculating the IHD and considering the number of hydrogen atoms present in relation to the maximum possible for a given number of carbon atoms. By understanding the degree of unsaturation, we can gain insights into the molecular structure and saturation of organic compounds.
Stay-at-Home Orders: Constitutional Rights or Government Overreach?
You may want to see also

Calculating the HDI (Index of Hydrogen Deficiency)
The Index of Hydrogen Deficiency (HDI) or Degrees of Unsaturation is a measure of the number of pi bonds and rings in a molecule. It is a useful tool for determining the structure of an unknown compound with a known molecular formula. The HDI is calculated using the formula:
> HDI = (No. of Rings + No. of pi bonds)
The HDI can also be calculated by comparing the number of hydrogen atoms in the compound to the expected number of hydrogen atoms based on the number of carbons in the corresponding alkane. The general formula for alkanes is given as CnH2n+2, where n is the number of carbon atoms.
For example, let's consider the compound C4H9Br. The expected number of hydrogen atoms is 10 (based on the alkane C4H10), but the actual number of hydrogen atoms is 9. Therefore, the HDI for this compound is 1.
It is important to note that the HDI is always a whole number, as each number represents a ring or pi bond. For instance, an HDI of 2 could represent two double bonds, two rings, one of each, or one triple bond. Additionally, the presence of certain elements, such as halogens or nitrogen, can affect the expected number of hydrogen atoms and, consequently, the HDI.
Checks and Balances: The Constitution's Core
You may want to see also
Explore related products

Drawing single-bond structures first
To determine the number of constitutional isomers, it is important to first understand what they are. Constitutional isomers are compounds that have the same molecular formula but different structures. In other words, they have the same type and number of atoms but differ in how these atoms are connected. For example, butane and isobutane are constitutional isomers with the molecular formula C4H10 but differing structures.
When determining the number of constitutional isomers for a given molecule, a systematic approach can be followed. Firstly, it is crucial to identify the molecular formula of the compound. This involves counting the number of each type of atom present. For instance, consider a molecule with the formula C3H6O.
The next step is to calculate the Hydrogen Deficiency Index (HDI) or the Index of Hydrogen Deficiency (IHD). This value corresponds to the number of double bonds or cyclic motifs in the molecule. The formula for calculating the HDI is: HDI = (2 * number of carbon atoms) + number of nitrogen atoms + 2 - (number of hydrogen and halogen atoms). Using the previous example of C3H6O, the HDI would be 1, indicating the presence of either a double bond or a cyclic structure.
With the HDI calculated, we can now start drawing possible single-bond structures for the constitutional isomers. It is important to ensure that the correct number of atoms is present in each structure and that bonding patterns follow established rules in organic molecules. For the C3H6O molecule, we can start by drawing linear structures with a central carbon atom bonded to two other carbon atoms and various hydrogen atoms to satisfy the molecular formula.
After exploring all possible linear structures, we can introduce branches to the core structure by substituting different atoms or functional groups. Finally, we can attempt to create cyclic structures by joining certain atoms within the chain or by substituting atoms within the ring. By following these steps, we can systematically generate the possible constitutional isomers for a given molecule while ensuring that we cover a wide range of structural possibilities.
Pinckney's Vote: Constitution's Fate in Charles' Hands?
You may want to see also

Naming molecules using IUPAC nomenclature rules
The IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). The purpose of the system is to give a unique and unambiguous name to each structure and to be able to compare each name with a unique and unambiguous structure. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created.
The IUPAC name of a compound can be written in the format: Locant + Prefix + Root + Locant + Suffix. The root indicates the total number of carbon atoms present in the longest carbon chain belonging to the compound. For example, 'meth' refers to a chain with one carbon atom, and 'pent' refers to a chain with five carbon atoms. The suffix in IUPAC nomenclature is usually a functional group belonging to the molecule, which follows the root of the name. It can be further divided into primary and secondary suffixes. A primary suffix is written immediately after the word root, as in the case of alkanes, where the suffix is 'ane'. A secondary suffix is generally written after the primary suffix. For example, compounds with an alkane and alcohol group attached to them are named 'alkanol', with 'ol' being the secondary suffix for the alcohol group.
Prefixes are added prior to the root of the compounds and are very useful in indicating the presence of side chains or substituent groups in the given organic molecule. These prefixes also offer insight into the cyclic or acyclic nature of the compounds. Primary prefixes indicate the cyclic or acyclic nature of the given compound. The prefix 'cyclo' is used for cyclic compounds. Secondary prefixes indicate the presence of side chains or substituent groups. For example, the 'CH3' group is called the methyl group.
Successive words are merged into one word (e.g., trimethyl heptane becomes trimethylheptane). Note that IUPAC uses one-word names throughout. This is why all parts are connected.
Some additional rules and examples are provided below:
- If there are multiple carboxyl groups on the same parent chain, multiplying prefixes are used. For example, malonic acid, CH2(COOH)2, is systematically named propanedioic acid.
- Alternatively, the suffix "-carboxylic acid" can be used in place of "oic acid", combined with a multiplying prefix if necessary – for example, mellitic acid is benzenehexacarboxylic acid.
- The common name for an aldehyde is derived from the common name of the corresponding carboxylic acid by dropping the word 'acid' and changing the suffix from '-ic' or '-oic' to '-aldehyde'.
- Alkenes are named for their parent alkane chain with the suffix "-ene" and a numerical root indicating the position of the carbon with the lower number for each double bond in the chain. For example, CH2=CHCH2CH3 is but-1-ene. Multiple double bonds take the form '-diene', '-triene', etc., with the size prefix of the chain taking an extra 'a'. For example, CH2=CHCH=CH2 is buta-1,3-diene.
- Simple cis and trans isomers may be indicated with a prefixed cis- or trans-. For example, cis-but-2-ene or trans-but-2-ene. However, cis- and trans- are relative descriptors. It is IUPAC convention to describe all alkenes using absolute descriptors of Z- (same side) and E- (opposite) with the Cahn–Ingold–Prelog priority rules.
- Alkynes are named using the same system, with the suffix "-yne" indicating a triple bond. For example, ethyne (acetylene) and propyne (methylacetylene).
- Halogen functional groups are prefixed with the bonding position and take the form of fluoro-, chloro-, bromo-, iodo-, etc., depending on the halogen.
Does the Constitution Still Work in Modern Times?
You may want to see also
Frequently asked questions
To determine the number of constitutional isomers, you must first ensure that the molecules have the same molecular formula. Then, you can count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index). If all the atoms are the same and the molecules have the same HDI, they are constitutional isomers.
Constitutional isomers are organic compounds that have the same molecular formula but differ in their structural connectivity. They are like two people wearing the same articles of clothing but in a different order.
The HDI is calculated by multiplying the number of carbon atoms by 2, adding the number of nitrogen atoms, adding 2, and then subtracting the number of hydrogen and halogen atoms.
The HDI tells you what kind of double bonds and rings are possible. For example, if the HDI = 1, the molecule must contain a double bond or a cycle, but not both.
There is no direct formula to calculate the exact number of constitutional isomers. However, there is a formula for the maximum number of possible stereoisomers, which is related to the number of chiral centres.































