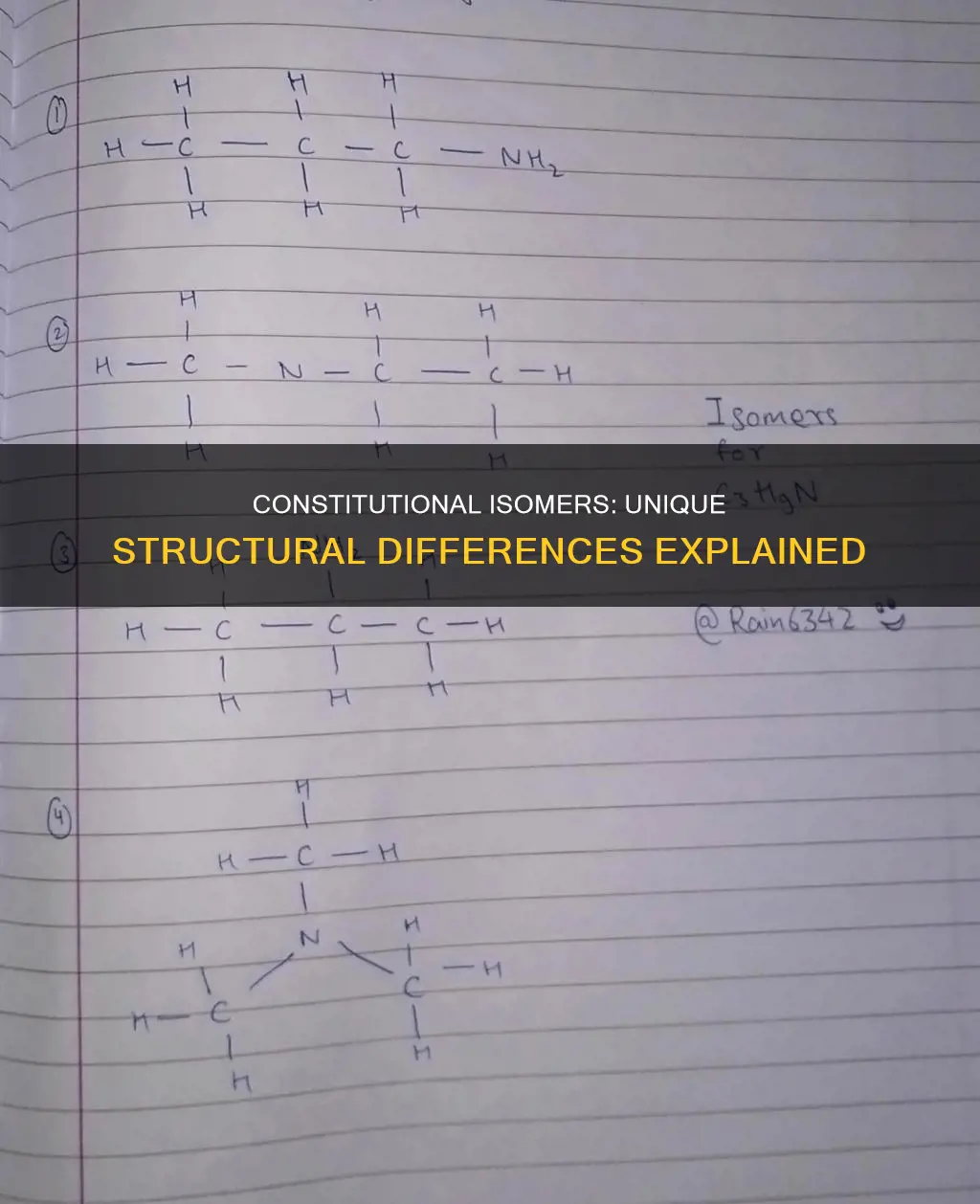
Constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but differ in the way their atoms are connected. They have the same number of atoms of each element, but their structures have a different arrangement of atoms and bonds. For example, ethanol and dimethyl ether are constitutional isomers because they both have the molecular formula C₂H₆O, but their atoms are connected differently. Stereoisomers, on the other hand, have the same molecular formula and the same connectivity of atoms, but differ in the arrangement of atoms in three-dimensional space. The two main types of stereoisomers are enantiomers and diastereomers, with enantiomers being non-superimposable mirror images of each other and diastereomers not being mirror images at all.
| Characteristics | Values |
|---|---|
| Molecular formula | Same |
| Connectivity of atoms | Different |
| Arrangement of atoms | Different |
| Number of atoms of each element | Same |
| Shape | Different |
| Properties | Different |
| Stability | Different |
| Reactivity | Different |
| Interaction with other molecules | Different |
Explore related products
What You'll Learn

Constitutional isomers have different atom connectivity
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the way their atoms are connected. This means that they have the same number of atoms of each element, but the structures have a different arrangement of atoms and bonds. For example, ethanol and dimethyl ether are constitutional isomers because they both have the molecular formula C₂H₆O, but their atoms are connected differently. In ethanol, the oxygen is bonded to a carbon and to a hydrogen, whereas in dimethyl ether, the oxygen is bonded to two carbons.
The difference in atom connectivity between constitutional isomers results in distinct physical and chemical properties, such as stability, reactivity, and interactions with other molecules. For instance, butane (C₄H₁₀) can exist as either a straight chain or a branched chain (isobutane). Both forms have the same number of carbon and hydrogen atoms, but their different connectivity leads to unique characteristics.
The concept of constitutional isomers highlights the importance of atomic connectivity in determining the properties of a molecule. While the number and type of atoms remain constant, altering the bonds between them can result in significant changes to the molecule's behaviour. This understanding is crucial in fields such as chemistry and pharmacology, where the manipulation of molecular structures can lead to the development of new materials or drugs.
It is worth noting that constitutional isomers are distinct from stereoisomers, which refer to molecules with the same molecular formula and atom connectivity but differing in the arrangement of atoms in three-dimensional space. Stereoisomers can be further classified into enantiomers and diastereomers. Enantiomers are non-superimposable mirror images of each other, while diastereomers are not mirror images and exhibit different chemical and physical properties, such as melting points and solubilities.
Understanding the Constitution's Intent
You may want to see also

Stereoisomers have the same atom connectivity
Constitutional isomers differ from one another in their atomic connectivity, i.e., the way their atoms are connected. For example, ethanol and dimethyl ether are constitutional isomers as they both have the molecular formula C₂H₆O, but their atoms are connected differently.
On the other hand, stereoisomers are isomers that have the same atom connectivity but differ in the way their atoms are arranged in space. They have the same molecular formula and the same order of attachment of atoms but differ in the way their atoms are oriented in space. Stereoisomers can be further classified into two types: conformational isomers and configurational isomers.
Conformational isomers, also called conforms, are stereoisomers that rapidly interconvert at room temperature. They are different spatial arrangements of the same compound. For example, anti and gauche conformers. Conformational isomers cannot be separated because they interconvert.
Configurational isomers, on the other hand, are stereoisomers that cannot interconvert unless covalent bonds are broken. They can be separated because they cannot interconvert. Configurational isomers can be further classified into two types: cis-trans isomers and isomers that contain asymmetric centers.
Cis-trans isomers refer to the arrangement of atoms or groups of atoms on either the same side or the opposite side of a double bond. Cis isomers have groups on the same side of the double bond, while trans isomers have groups on opposite sides.
Asymmetric centers, also called chiral or stereogenic centers, are atoms bonded to four different groups. Interchanging two groups at a chiral center can produce a stereoisomer. For example, interchanging groups at an asymmetric center can convert a cis isomer to a trans isomer or vice versa.
Stereoisomers that are mirror images of each other and are not superimposable, even though the atomic distances are the same, are called enantiomers. Enantiomers have same physical properties and cannot be separated by physical methods. They are characterized using the symbols R and S. Diastereomers are stereoisomers that are not enantiomers, meaning they are not mirror images of each other.
Understanding Differences in Excerpts Through Comparative Analysis
You may want to see also

Enantiomers are non-superimposable mirror images
Constitutional isomers differ in the way their atoms are connected. For example, ethanol and dimethyl ether are constitutional isomers because they both have the molecular formula C₂H₆O, but their atoms are connected differently.
Enantiomers are a type of stereoisomer. Stereoisomers are isomers that have the same connectivity but differ in the way their atoms are arranged in space. Enantiomers are defined as a pair of stereoisomers that are non-superimposable mirror images of one another. This means they are molecules made up of identical atoms, bonded together in the same way, but the 3D arrangement of the atoms in enantiomers is different, as these molecules are mirror images of each other.
You cannot superimpose one enantiomer onto the other without breaking and remaking bonds. Enantiomers are optically active and exhibit equal and opposite light rotation. A 50:50 mixture of a pair of enantiomers is called a racemic mixture. This is optically inactive since the rotations produced by each of the enantiomers cancel each other out.
The most common type of "chirality" is observed when a carbon atom has four different groups attached to it (so it must be sp3 hybridised). This carbon atom is then described as a chirality center. An object that has a non-superimposable mirror image has the property of handedness and is said to be chiral. One that has a superimposable mirror image is achiral (it doesn't have "handedness"). This applies to your left and right hands, which are essentially mirror images but are not superimposable.
Understanding California's Hostile Work Environment Laws
You may want to see also
Explore related products

Diastereomers are not mirror images
Constitutional isomers differ in the way their atoms are connected. For example, ethanol and dimethyl ether are constitutional isomers because they both have the molecular formula C₂H₆O, but their atoms are connected differently.
Constitutional isomers are not stereoisomers, which are isomers with the same connectivity but a different arrangement of their atoms in space. Stereoisomers include two types: conformational isomers and configurational isomers. Conformational isomers, also called conformers, are stereoisomers that rapidly interconvert at room temperature and, therefore, cannot be separated. Configurational isomers, on the other hand, are stereoisomers that cannot interconvert unless covalent bonds are broken and, thus, can be separated. Configurational isomers include two types: cis-trans isomers and isomers that contain asymmetric centers.
Configurational isomers are also called stereoisomers. Stereoisomers with more than one stereocenter that are non-superimposable non-mirror images of each other are called diastereomers. Diastereomers are not mirror images of each other. They are optically active isomers but not mirror images. Enantiomers, on the other hand, are stereoisomers that are non-superimposable mirror images of each other.
To summarise, constitutional isomers differ in their atomic connectivity, while stereoisomers differ in the arrangement of their atoms in space. Diastereomers are a type of stereoisomer that are not mirror images of each other, in contrast to enantiomers, which are stereoisomers that are mirror images.
Congress Powers: Understanding the Limits and Boundaries
You may want to see also

Configurational isomers can be separated
Constitutional isomers differ in the way their atoms are connected. For example, ethanol and dimethyl ether are constitutional isomers because they both have the molecular formula C₂H₆O, but their atoms are connected differently.
Configurational isomers, on the other hand, are stereoisomers that cannot interconvert unless covalent bonds are broken. They can be separated because they do not interconvert. There are two types of configurational isomers: diastereomers and enantiomers. Enantiomers are non-superposable mirror images. For example, 2,4-hexadiene has three different configurations, which are designated as trans-trans, cis-cis, and trans-cis. Because the two ends of this molecule are identically substituted, the trans-cis becomes identical with cis-trans.
Chiral molecules (optical isomers) can be identified by whether a molecule and its mirror image are not identical. A pair of molecules that are not identical but are mirror images of each other are called enantiomers. Diastereomers are another type of configurational isomer that can be separated by normal physical methods such as distillation, recrystallisation, and chromatography. They have different physical properties, such as boiling point, melting point, and solubility.
Enantiomers can also be separated through the resolution of a racemic mixture. A racemic mixture is a mixture of equal amounts of both enantiomers, and the separation of this mixture into its component enantiomers is called a resolution. For example, Pasteur found that meso-tartaric acid could be easily separated from racemic-tartaric acid due to its different physical properties, such as solubility.
Understanding the Foundation: Key Principles of the Constitution
You may want to see also
Frequently asked questions
Constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but differ in the connectivity of their atoms.
Constitutional isomers differ from stereoisomers in the way their atoms are connected. Constitutional isomers have a different arrangement of atoms within the molecules, while stereoisomers have the same atom connectivity but differ in the arrangement of atoms in three-dimensional space.
Yes, ethanol and dimethyl ether are constitutional isomers. They both have the molecular formula C₂H₆O, but their atoms are connected differently. The oxygen in ethanol is bonded to a carbon and a hydrogen, while the oxygen in dimethyl ether is bonded to two carbons.
Other types of isomers include stereoisomers, which can be further divided into enantiomers and diastereomers. Enantiomers are stereoisomers that are non-superimposable mirror images of each other, while diastereomers are not mirror images and can have different chemical and physical properties.
Yes, another type of stereoisomer is a conformational isomer (also known as a conformer). Conformational isomers are stereoisomers that rapidly interconvert at room temperature and, therefore, cannot be separated. They are different spatial arrangements of the same compound.