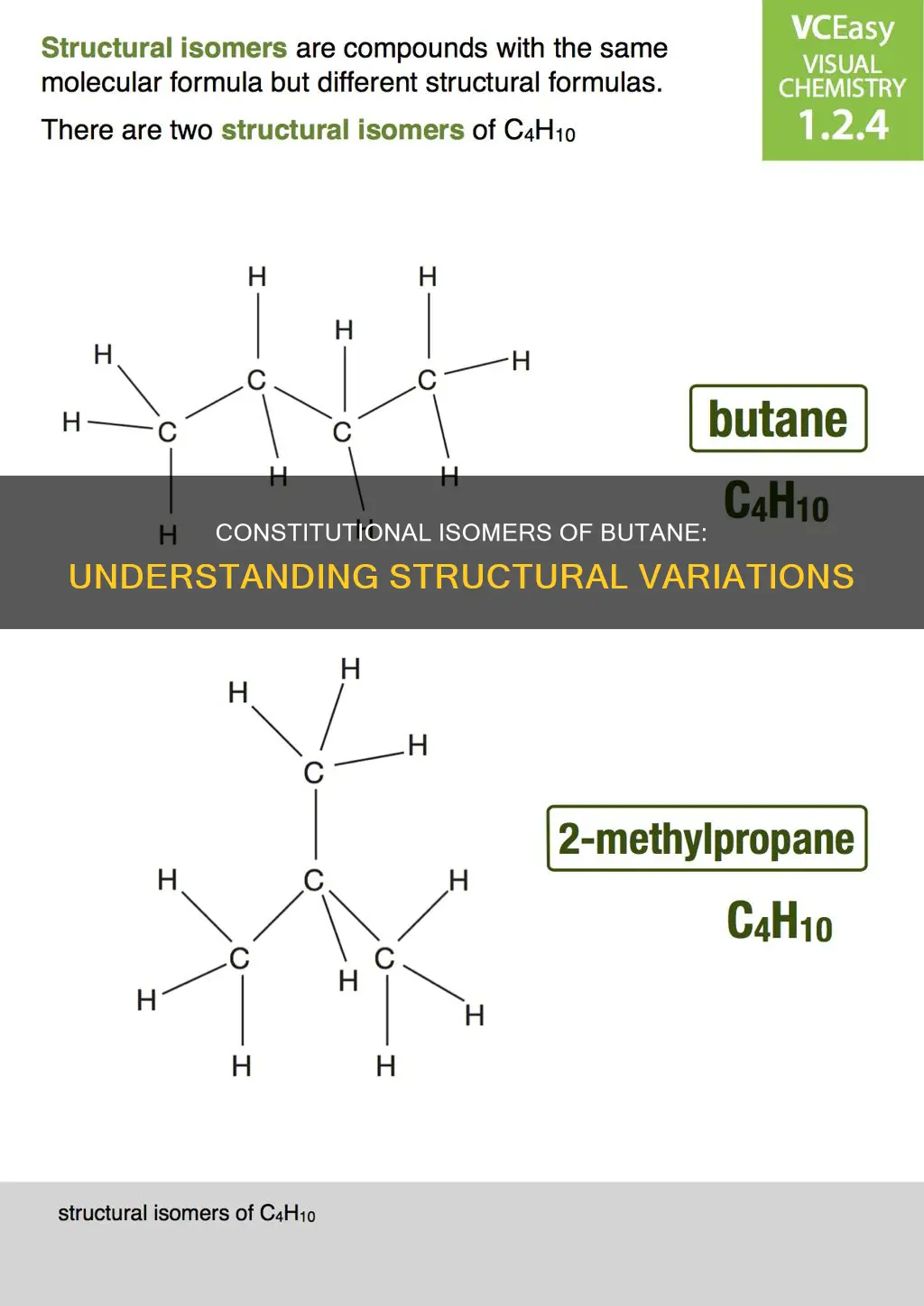
Butane is a highly flammable, colourless, and easily liquefied gas with a wide range of applications, from fuel for portable stoves to manufacturing processes. It exhibits isomerism, a phenomenon where molecules with the same molecular formula have different chemical and physical properties due to variations in the arrangement of atoms. Butane has two constitutional isomers: n-butane, a straight-chain compound with four carbon atoms, and isobutane (2-methylpropane), which has a branched structure. These isomers differ in their connectivity of atoms, resulting in distinct characteristics despite sharing the same molecular formula, C4H10. Understanding the concept of constitutional isomers is fundamental in organic chemistry, as it highlights how subtle changes in molecular structure can lead to significant differences in properties.
| Characteristics | Values |
|---|---|
| Constitutional isomers of butane | 2-methylpropane (also known as isobutane) |
| Molecular formula of butane | C₄H₁₀ |
| Molecular formula of 2-methylpropane | C₄H₁₀ |
| Structural difference | Butane is a straight-chain alkane with four carbon atoms, while 2-methylpropane is branched with the central carbon atom connected to three other carbon atoms |
| Number of covalent bonds | 13 |
| Types of isomerism | Structural or constitutional isomerism, and stereoisomerism or spatial isomerism |
Explore related products
What You'll Learn

2-methylpropane is the constitutional isomer of butane
Butane is a straight-chain alkane with four carbon atoms bonded with single covalent bonds. It has two isomers: n-butane and isobutane, also known as 2-methylpropane. These two molecules are constitutional isomers, meaning they share the same molecular formula (C4H10) but differ in their structure or spatial arrangement of atoms. In butane, all carbon atoms are in a straight chain, while isobutane has a side chain in its molecule.
Constitutional isomers, also known as structural isomers, are compounds that have the same molecular formula but different structural formulas. In other words, they have a different connectivity of atoms or groups of atoms in their molecules. This means that the atoms are bonded differently, resulting in distinct chemical structures. In the case of butane and isobutane, they have the same number of carbon and hydrogen atoms, but the arrangement of these atoms differs. Butane has an unbranched chain of four carbon atoms, while isobutane has three carbon atoms in a row and the fourth carbon atom is attached as a branch to the middle carbon atom. This structural difference leads to varying properties, such as different chemical and physical properties, despite having the same molecular formula.
The concept of isomerism is important in organic chemistry. Isomerism occurs when two or more compounds have the same chemical formula but different chemical structures and properties. These compounds, known as isomers, can exhibit structural or constitutional isomerism, where the bonds between the atoms differ, or stereoisomerism or spatial isomerism, where the bonds are the same but the relative positions of the atoms differ. Stereoisomers, which are a type of conformational isomer, can be converted into one another by rotation around a single bond. Alkanes, such as butane and isobutane, typically show conformational isomerism due to the presence of C-C bonds. For example, when the butane molecule is rotated at the axis of the C-C bond, different conformations like eclipsed, gauche, and anti butane conformational isomers are formed.
In summary, 2-methylpropane, also known as isobutane, is the constitutional isomer of butane. They share the same molecular formula but differ in their structural arrangement, with butane having an unbranched chain and isobutane having a branched chain. This difference in structure leads to distinct chemical and physical properties between the two compounds.
Justice in the Constitution: An Example
You may want to see also

Isobutane is a structural isomer of butane
Isobutane, also known as 2-methylpropane, is a structural isomer of butane. Butane has two isomers: n-butane and isobutane. These molecules have the same molecular formula, C4H10, but differ in their connectivity of atoms, making them constitutional isomers.
N-butane is a straight-chain compound with four carbon atoms bonded by single covalent bonds. In contrast, isobutane has a branched structure, with three carbon atoms in a chain and one carbon atom as a side chain attached to the second carbon atom in the chain. This structural difference leads to varying physical and chemical properties, despite the two molecules having the same molecular formula.
The phenomenon of isomerism occurs when two or more compounds have the same chemical formula but different chemical structures. Isomers are chemical compounds with identical chemical formulas but distinct characteristics and atom arrangements in the molecule. Butane and isobutane are constitutional isomers, a type of isomerism where the bonds between the atoms differ, resulting in different structural formulas.
Conformational isomers, another type of isomer, are isomers that differ in their conformation or spatial arrangement. Butane can form conformational isomers through rotation around the C-C single bond, resulting in eclipsed, gauche, and anti conformations. The eclipsed conformation is unstable due to the presence of identical groups directly in line with each other, while the gauche conformation is more stable, with these groups positioned at 60 degrees from each other. The anti conformation, with groups at 180 degrees from each other, is the most stable form.
In summary, isobutane is a structural isomer of butane, with a branched structure differing from the straight-chain structure of n-butane. This isomerism results in distinct physical and chemical properties, highlighting the importance of structural arrangements in organic chemistry.
Constitution Party: A Membership Overview
You may want to see also

Butane has two isomers
N-butane, also known as normal butane, has a linear or straight-chain structure with four carbon atoms bonded by single covalent bonds. It has the chemical formula CH3-CH2-CH2-CH3. On the other hand, isobutane has a branched structure. It consists of three carbon atoms (C1, C2, and C3) joined in a straight chain and one side chain or branch formed by C2 and C4. This structural difference leads to varying physical and chemical properties, despite the isomers having the same molecular formula.
Constitutional isomers, such as n-butane and isobutane, share the same chemical formula but differ in the connection of their atoms or groups of atoms. They have different structural formulas, also known as structural isomerism. In other words, constitutional isomers have a different spatial arrangement of atoms in their molecules, affecting their properties. This classification is a fundamental concept in organic chemistry.
Stereoisomers, another type of isomer, have the same molecular formula and bonding arrangement but differ in the relative positions of their atoms in space, known as spatial or stereoisomerism. Butane also exhibits stereoisomerism, with conformational isomers that differ in their conformation based on rotation around a single bond. These conformational isomers include eclipsed, gauche, and anti butane.
In summary, butane has two isomers, n-butane and isobutane, which differ structurally and in their properties. These isomers are constitutional isomers, with the same molecular formula but different connectivity of atoms. Butane also demonstrates stereoisomerism through conformational isomers formed by rotation around a single bond.
Where is the US Constitution?
You may want to see also
Explore related products

Constitutional isomers have the same molecular formula
Constitutional isomers are chemical compounds with identical chemical formulas but different characteristics and atom arrangements in the molecule. They have the same molecular formula but different structural arrangements of atoms in their molecules. In other words, they have the same formula but different connectivity.
For example, butane and isobutane are constitutional isomers. Butane has four carbon atoms bonded in a continuous chain, whereas isobutane has three carbon atoms from the parent chain and one carbon atom placed as the side chain at C-2 of the parent chain. They have different connectivity of atoms and are constitutional isomers of each other.
Another example is ethanol (ethyl alcohol) and dimethyl ether, which both have the formula C2H6O. They have the same atoms in the same ratios, but the connections between those atoms are different, making them constitutional isomers.
Constitutional isomers can have the same or different functional groups. For instance, two molecules can have the same functional group (OH) located at different points on the carbon skeleton, making them constitutional isomers.
When identifying constitutional isomers, it is important to first look at the molecular formula. From there, the HDI can be determined, which gives an idea of the structural features to look for in the molecule.
Benjamin Rush's Medical Freedom Vision for the Constitution
You may want to see also

Stereoisomers are a type of isomer
Stereoisomers are molecules that have the same molecular formula and sequence of bonded atoms (or constitution), but differ in the three-dimensional orientations of their atoms in space. This means that stereoisomers have the same connectivity but differ in their spatial arrangement. They are a form of isomerism, which is a phenomenon where two or more compounds have the same chemical formula but different chemical structures.
There are two main forms of isomerism: structural or constitutional isomerism, and stereoisomerism or spatial isomerism. Constitutional isomers have the same molecular formula but different connectivities, or structural arrangements. Stereoisomers, on the other hand, have the same connectivity but differ in their spatial arrangement.
An example of a stereoisomer is the pair of cis- and trans-2-butene, which are also known as diastereomers. These molecules are not mirror images of each other, and they seldom have the same physical properties. Another example is the pair of enantiomers, which are stereoisomers that are related to each other by a reflection. They are mirror images of each other that are non-superposable, similar to the left and right hands.
In the context of butane, the given options are: 3-ethyl-3-methylpentane, 3,3-dimethylpentane, 2-methylpropane, and 2,3,4-trimethylpentane. Among these, 2-methylpropane is identified as the constitutional isomer of butane because it has the same molecular formula (C4H10) but a different spatial arrangement of atoms. The other options have different molecular formulas and are not isomers of butane.
Ending Constitution Revision: Why and How?
You may want to see also











































