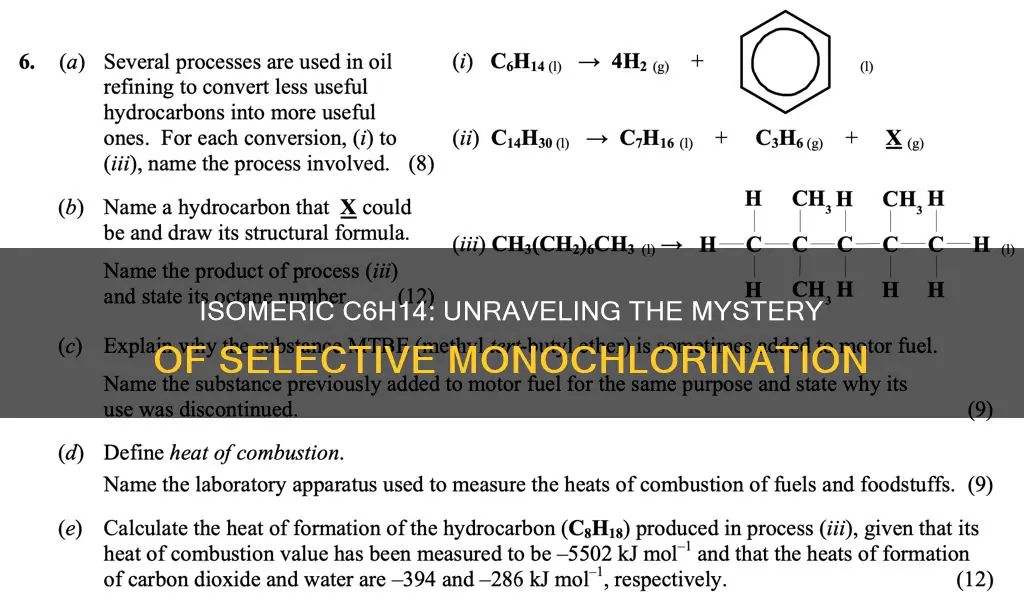
The molecular formula C6H14 represents hexane and its isomers. There are five constitutional isomers with this formula, and when treated with chlorine, they give different numbers of monochlorination products. For example, treating isomer A with chlorine at 300°C yields two monochlorination products, while isomer B gives five. The constitutional isomer of C6H14 that gives only two monochlorination products is 2,2-dimethylbutane, due to its unique structural arrangement of atoms.
| Characteristics | Values |
|---|---|
| Constitutional isomer of C6H14 that gives only two monochlorination products | 2,2-Dimethylbutane |
| Other possible isomers | 2-Methylpentane, 3-Methylpentane, 2,3-Dimethylbutane |
| Number of constitutional isomers with the molecular formula C6H14 | 5 |
| Reason for 2,2-Dimethylbutane giving only two monochlorination products | Only two unique types of hydrogen atoms that can be replaced by chlorine atoms |
Explore related products
What You'll Learn
- ,2-Dimethylbutane has two types of hydrogen atoms that can be replaced by chlorine
- ,3-Dimethylbutane has three types of hydrogen atoms, leading to multiple chlorination products
- Methylpentane has multiple hydrogen atoms that can give rise to multiple chlorination products
- Methylpentane has a variety of hydrogen atoms that can form several chlorinated products
- methylpropane has a unique structural arrangement of atoms

2,2-Dimethylbutane has two types of hydrogen atoms that can be replaced by chlorine
The constitutional isomer of C6H14 that gives only two monochlorination products is 2,2-Dimethylbutane. This is due to its unique structural arrangement, which results in only two types of hydrogen atoms that can be replaced by chlorine.
Firstly, it is important to understand the structure of 2,2-Dimethylbutane. This isomer is a branched alkane, with a base structure of butane, which consists of a straight chain of four carbon atoms. The "2,2-dimethyl" prefix indicates that there are two methyl (CH3) groups attached to the second carbon atom in the butane chain. This central carbon atom, also known as a tertiary carbon, is attached to three other carbon atoms.
Now, let's discuss the two types of hydrogen atoms in 2,2-Dimethylbutane that can be replaced by chlorine:
- Hydrogen Atoms on the Central Carbon Atom: The central carbon atom in 2,2-Dimethylbutane is attached to a hydrogen atom. During chlorination, this hydrogen atom can be replaced by a chlorine atom. This substitution results in the formation of 2-chloro-2-methylbutane.
- Hydrogen Atoms on the Methyl Groups: Each of the two methyl groups (CH3) in 2,2-Dimethylbutane has three hydrogen atoms attached to it. These hydrogen atoms are equivalent, meaning that replacing any one of them with chlorine will give the same product. The resulting compound is 2-chloro-2,2-dimethylbutane.
The symmetry of 2,2-Dimethylbutane is crucial to understanding why it gives only two monochlorination products. While there are multiple hydrogen atoms available for substitution, the symmetric structure means that some of these hydrogens are equivalent and will result in the same product when replaced by chlorine. This symmetry reduces the number of unique structures that can be formed.
In conclusion, 2,2-Dimethylbutane has two types of hydrogen atoms that can be replaced by chlorine: those on the central carbon atom and those on the equivalent methyl groups. This unique structural arrangement leads to only two distinct monochlorination products, making it the only isomer of C6H14 with this property.
Factual Judgments: Restating the Obvious?
You may want to see also

2,3-Dimethylbutane has three types of hydrogen atoms, leading to multiple chlorination products
The molecular formula C6H14 represents hexane and its isomers. 2,3-Dimethylbutane is one such isomer of hexane. It has three types of hydrogen atoms, leading to multiple chlorination products. The monochlorinated products of 2,3-dimethylbutane reacting with chlorine gas (Cl2) and light arise from the reaction of light energy breaking the Cl2 molecule into highly reactive Cl atoms that replace the hydrogen atoms in the hydrocarbon molecule. The monochlorinated products can be: 1-chloro-2,3-dimethylbutane, 2-chloro-2,3-dimethylbutane, and 3-chloro-2,3-dimethylbutane.
The mechanism of formation involves the initial abstraction of hydrogen by a chlorine radical, followed by the combination of the resulting alkyl radical with another chlorine atom. The overall reaction demonstrates how radical chlorination can lead to multiple products, specifically considering the structure of the original alkane. Since chlorine is a less selective halogen compared to bromine, we can expect a mixture of different products.
On the other hand, the isomer of C6H14 that gives only two monochlorination products is 2,2-Dimethylbutane. This is because 2,2-Dimethylbutane has only two unique types of hydrogen atoms that can be replaced by a chlorine atom to give different monochlorination products. One type is the hydrogen on the central carbon atoms, and the other is the hydrogens on the methyl groups. Since all methyl groups are equivalent, replacing any of these hydrogens gives the same product. Similarly, since the two central carbon atoms are equivalent, they also yield the same product upon chlorination.
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the spatial arrangement of atoms in their molecules. For example, n-butane has an unbranched chain, meaning none of its carbon atoms are bonded to more than two other carbon atoms. In contrast, the compound 2-methylpropane, another isomer of C6H14, has a branched chain, indicating that the carbon atom in the centre of the Lewis structure is bonded to three other carbon atoms.
Lincoln's Constitutional Conundrum: Ignoring the Fundamentals
You may want to see also

2-Methylpentane has multiple hydrogen atoms that can give rise to multiple chlorination products
The molecular formula C6H14 represents hexane and its isomers. 2-methylpentane is one such isomer of hexane. It has multiple types of hydrogen atoms available for substitution, leading to several possible monochlorination products when reacted with chlorine.
Constitutional isomers, also referred to as structural isomers, possess the same molecular formula but differ in the spatial arrangement of atoms in their molecules. In the case of 2-methylpentane, there are multiple hydrogen atoms that can give rise to multiple chlorination products. This is because 2-methylpentane has a branched chain structure, which allows for a variety of hydrogen atom substitutions.
The process of chlorination involves replacing one hydrogen atom in the molecule with a chlorine atom. This reaction typically occurs via a free radical mechanism, and the products depend on the different types of hydrogen atoms present in the molecule. During chlorination, chlorine radicals abstract hydrogen atoms from 2-methylpentane, leading to the formation of various monochlorinated products.
The specific monochlorinated products that form depend on the unique positions of chlorination. For example, in the case of pentane, there are three types of hydrogens based on the carbon they are attached to: primary hydrogens (on the terminal carbons), secondary hydrogens (on the carbons adjacent to the terminal carbons), and tertiary hydrogens (if present, but pentane does not have tertiary hydrogens). Replacing one hydrogen atom at each unique position with a chlorine atom results in three distinct products: 1-chloropentane, 2-chloropentane, and 3-chloropentane.
The number and variety of chlorination products depend on the structure of the molecule and the availability of different types of hydrogen atoms for substitution. In the case of 2-methylpentane, its branched chain structure and multiple types of hydrogen atoms allow for a greater number of chlorination products compared to other isomers of hexane.
Unlocking Constitution Island's Secrets: The Password
You may want to see also
Explore related products

3-Methylpentane has a variety of hydrogen atoms that can form several chlorinated products
The molecular formula C6H14 represents hexane and its isomers. 3-Methylpentane is one such isomer of C6H14. It has a variety of hydrogen atoms that can form several chlorinated products.
When 3-methylpentane undergoes chlorination, hydrogen atoms are replaced by chlorine atoms. This process can occur at different positions on the carbon chain, leading to multiple chlorinated products. Specifically, there are five carbon atoms in 3-methylpentane where chlorine atoms can replace hydrogen atoms. This results in a total of 15 different dichlorinated products.
The formation of these products can be understood by examining the structure of 3-methylpentane. The molecule has five carbon atoms, labelled from C-1 to C-5. Chlorination can occur at any of these positions, leading to multiple combinations of chlorine atom placements. For example, chlorinating C-2 and C-3 simultaneously results in a product known as 2,3-dichloro-3-methylpentane.
The variety of hydrogen atoms in 3-methylpentane allows for the formation of several chlorinated products. This is similar to other isomers of C6H14, such as 2,3-dimethylbutane and 2-methylpentane, which also have multiple types of hydrogen atoms available for substitution. However, the isomer 2,2-dimethylbutane is unique in that it has only two different types of hydrogen atoms that can be substituted by chlorine, leading to only two monochlorination products.
In summary, 3-methylpentane has a variety of hydrogen atoms that enable the formation of several chlorinated products through substitution reactions with chlorine atoms. This results in a range of isomeric compounds, showcasing the complex nature of chlorination reactions involving alkanes.
Supreme Court: Constitutional Overreach or Justified Action?
You may want to see also

2-methylpropane has a unique structural arrangement of atoms
The constitutional isomer of C6H14 that gives only two monochlorination products is 2,2-Dimethylbutane. This is because 2,2-Dimethylbutane has only two unique types of hydrogen atoms that can be replaced by a chlorine atom to give different monochlorination products. One type is the hydrogen on the central carbon atom, and the other is the hydrogen on the methyl groups.
Now, let's talk about 2-methylpropane and its unique structural arrangement of atoms. 2-methylpropane, also known as isobutane, is a structural isomer of butane (C4H10). It has a branched-chain structure, in contrast to the straight-chain structure of butane. In 2-methylpropane, there is a side chain in the molecule, with three carbon atoms in a row and the fourth carbon attached as a branch. This structural difference means that 2-methylpropane has a more compact, spherical-like shape compared to butane.
The unique structural arrangement of 2-methylpropane has implications for its physical and chemical properties. Due to its compact and branched structure, 2-methylpropane has a reduced surface area in contact with neighbouring molecules. This leads to weaker London dispersion forces compared to butane. As a result, 2-methylpropane has a lower boiling point than butane.
The way atoms are bonded and arranged within a molecule affects its strength and the types of forces it can exert or experience. The branched structure of 2-methylpropane, with its carbon backbone and side chain, gives rise to distinct properties that differentiate it from its straight-chain isomer, butane.
In summary, 2-methylpropane, or isobutane, exhibits a unique structural arrangement of atoms with a branched carbon chain and a side chain. This structural arrangement results in a more compact molecule with weaker intermolecular forces and a lower boiling point compared to its straight-chain isomer, butane. The distinct atomic arrangement of 2-methylpropane highlights the complexity and diversity within organic molecules, demonstrating the importance of molecular structure in determining a compound's properties.
Congressional Powers Denied: Constitutional Limits Explored
You may want to see also
Frequently asked questions
2,2-Dimethylbutane.
One product is from replacing a hydrogen on a central carbon atom, and the other is from replacing a hydrogen on a methyl group.
There are five constitutional isomers with the molecular formula C6H14.
The five isomers are:
- Hexane
- 2-Methylpentane
- 3-Methylpentane
- 2,2-Dimethylbutane
- 2,3-Dimethylbutane
It has a branched chain, with the carbon atom in the centre of the Lewis structure bonded to three other carbon atoms.


































