Cyclohexene is a derivative of cyclohexane, a cyclic organic compound composed of six carbon atoms bonded together. Isomers are compounds that contain atoms or groups of atoms bonded to the cyclohexane ring itself. Cyclohexane and 1-hexene are not constitutional isomers because they have different structural arrangements. However, 3-methylpent-2-ene is a structural isomer of cyclohexane. In this response, we will explore the topic of which compound is a constitutional isomer of cyclohexene.
| Characteristics | Values |
|---|---|
| Name of the isomer | 3-methylpent-2-ene |
| Molecular formula | C6H12 |
| Number of carbon atoms | 6 |
| Structure | Straight chain with a double bond and a methyl group |
| Connectivity | Different from cyclohexane due to the presence of a carbon-carbon double bond and a methyl group |
Explore related products
What You'll Learn

Constitutional isomers have the same formula but different atom connections
Constitutional isomers, also known as structural isomers, are compounds that have the same molecular formula but differ in the way their atoms are connected or arranged. In other words, they have the same number of atoms but a different connectivity of atoms in the molecule. For example, 1,2-dichlorocyclohexane and 1,4-dichlorocyclohexane are constitutional isomers of cyclohexane derivatives as they share the same chemical formula but differ in the arrangement of their atoms.
Constitutional isomers can be distinguished from each other by counting the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index or HDI). If all the atoms are the same and the molecules have the same HDI, then they are constitutional isomers. The HDI value is determined by the combination of cycles and double or triple bonds in a molecule. For instance, butane (C4H10) has two constitutional isomers: n-butane (a straight chain) and isobutane (a branched chain). Both have four carbons and ten hydrogens, but they are connected differently, making them constitutional isomers.
Another example of constitutional isomers is ethanol (C2H6O) and dimethyl ether (C2H6O). Both molecules have the same atoms in the same ratios, but the connections between those atoms differ, making them constitutional isomers. Similarly, cyclohexane (C6H12) and 3-methylpent-2-ene (C6H12) are constitutional isomers. Cyclohexane has a cyclic structure, while 3-methylpent-2-ene has a straight-chain structure with a double bond and a methyl group, resulting in a different arrangement of atoms.
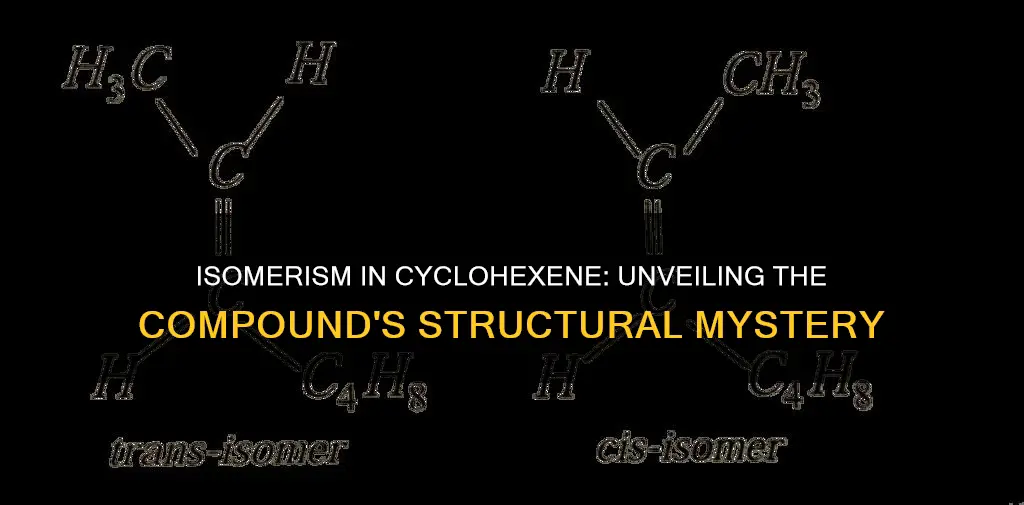
It is important to note that stereoisomers and constitutional isomers are distinct. Stereoisomers have the same molecular formula and chemical bonds between atoms but differ in the spatial (three-dimensional) orientation of atoms. For instance, cis-1,2-dihydroxycyclohexane and trans-1,2-dihydroxycyclohexane are stereoisomers of cyclohexane-1,2-diol. In the cis isomer, the substituent groups are on the same plane, while in the trans isomer, they are on opposite planes.
Frederick Douglass' View of the Constitution
You may want to see also

Cyclohexane and 1-hexene are not isomers due to different structures
While cyclohexane and 1-hexene share the same molecular formula, C6H12, they are not isomers. This is because they have different structural arrangements. Cyclohexane is a cyclic compound, with its carbon atoms forming a ring structure. On the other hand, 1-hexene is a linear alkene with a double bond between the first and second carbon atoms in the chain.
Constitutional isomers, also known as structural isomers, have the same molecular formula but different arrangements of atoms within their structures. For example, 1,2-dichlorocyclohexane and 1,4-dichlorocyclohexane are constitutional isomers of cyclohexane derivatives. They have the same chemical formula but differ in the way their chlorine atoms are bonded to the ring. Another example is butane (C4H10), which has two constitutional isomers: n-butane (a straight chain) and isobutane (a branched chain).
Stereoisomers, on the other hand, have the same molecular formula and chemical bonds between atoms but differ in the spatial (three-dimensional) orientation of atoms. For instance, cis-1,2-dihydroxycyclohexane and trans-1,2-dihydroxycyclohexane are stereoisomers of cyclohexane. In the cis form, the two alcohol groups are bonded on the same plane of the ring, while in the trans form, these groups are present on different planes of the ring.
Cyclohexane and 1-hexene, however, do not fall into either of these categories of isomers. This is because, despite having the same chemical formula, they have distinct structures. Basic organic chemistry principles dictate that cyclic structures and linear alkenes cannot be classified under the same isomer category.
Therefore, cyclohexane and 1-hexene are not isomers due to their different structures. They are, in fact, different types of hydrocarbons.
Understanding Iowa's Hostile Work Environment Laws
You may want to see also

Cyclohexane is cyclic, 1-hexene is linear
In organic chemistry, a structural isomer, also known as a constitutional isomer, is a compound that has the same molecular formula as another compound but a different arrangement of atoms. Cyclohexane, which has the molecular formula C6H12, is a cyclic alkane consisting of six carbon atoms arranged in a ring. On the other hand, 1-hexene, an alkene, has the same molecular formula, C6H12, but differs from cyclohexane in terms of its structure, as it is a linear compound with a double bond between two of its carbon atoms.
Constitutional isomers have the same molecular formula but different arrangements of atoms. For instance, the constitutional isomers of cyclohexane derivatives are 1,2-dichlorocyclohexane and 1,4-dichlorocyclohexane. Stereoisomers, on the other hand, have the same molecular formula and chemical bonds between atoms but differ in the spatial (three-dimensional) orientation of atoms. When substituent groups are present on the same plane, it is considered a cis isomer, whereas when the groups are on opposite planes, it is classified as a trans isomer.
Cyclohexane and 1-hexene cannot be classified as constitutional isomers because they have different structural arrangements. Cyclohexane is a cyclic compound, while 1-hexene is linear. This distinction is fundamental in organic chemistry principles, where cyclic structures and linear alkenes cannot be categorized as the same type of isomer, despite sharing the same chemical formula.
The difference between cyclohexane and 1-hexene lies in their molecular structures. Cyclohexane is a cyclic alkane with a molecular formula of C6H12, meaning it has six carbon atoms bonded together to form a ring structure. This ring structure gives cyclohexane its unique properties, including a non-planar conformation that bends and twists to form a stable chair-like shape. On the other hand, 1-hexene is a linear alkene with the same molecular formula, C6H12, but a different arrangement of atoms. Specifically, 1-hexene has a double bond between the first and second carbon atoms in its chain structure, making it acyclic.
While cyclohexane and 1-hexene share the same molecular formula, they are not considered constitutional isomers due to their distinct structural characteristics. Cyclohexane, with its cyclic nature, forms a ring structure, while 1-hexene, being a linear compound, exhibits a straight-chain configuration with a double bond. These structural disparities set them apart, classifying them as different types of hydrocarbons rather than isomers of each other.
Border Fence: Constitutional or Unconstitutional?
You may want to see also
Explore related products
$179.21

Cyclohexane has no double bonds, 1-hexene does
Cyclohexane and cyclohexene are both hydrocarbons composed entirely of hydrogen and carbon atoms. However, they differ in their structural arrangements. Cyclohexane is a saturated hydrocarbon, meaning all the bonds between carbon atoms and hydrogens are single. It is a cyclic compound with a molecular formula of C6H12, forming a ring structure. On the other hand, cyclohexene is an unsaturated hydrocarbon, meaning it has a double bond between two carbon atoms. This double bond makes it more reactive than cyclohexane. Cyclohexene is also a cycloalkene, a type of organic compound with one or more double bonds between carbon atoms in its ring structure. Its chemical formula is C6H10.
Now, let's compare cyclohexane to 1-hexene. Cyclohexane and 1-hexene have the same molecular formula, C6H12, but they differ in their structural arrangements. Cyclohexane is a cyclic compound, meaning its carbon atoms form a ring structure. 1-hexene, on the other hand, is a linear alkene with a double bond between the first and second carbon atoms in its chain structure. Due to these differences in their structures, cyclohexane and 1-hexene are not considered constitutional isomers. Constitutional isomers, also known as structural isomers, are compounds that have the same molecular formula but different arrangements of atoms within their structures.
To better understand the concept of constitutional isomers, let's look at an example. Butane (C4H10) has two constitutional isomers: n-butane and isobutane. N-butane is a straight-chain structure, while isobutane is a branched-chain structure. Both compounds have the same molecular formula but differ in their structural arrangements, making them constitutional isomers.
In summary, cyclohexane has no double bonds, while 1-hexene has a double bond between its first and second carbon atoms. This difference in structural arrangements means that cyclohexane and 1-hexene are not considered constitutional isomers.
Presidential Cabinet: Examples and Their Roles
You may want to see also

2-methylhexane is a structural isomer of cyclohexane
In organic chemistry, a structural isomer, also known as a constitutional isomer, is a compound that has the same molecular formula as another compound but a different arrangement of atoms. Cyclohexane, which has the molecular formula C6H12, is a cyclic alkane consisting of six carbon atoms bonded together in a ring. To find structural isomers of cyclohexane, we need to consider compounds that also have six carbon atoms but differ in structure.
Considering cyclohexane, 2-methylhexane is a structural isomer as it contains the same carbon count but with a different structural layout. It has the molecular formula C7H16, which is different from cyclohexane's formula C6H12. Therefore, 2-methylhexane is not an isomer of cyclohexane. However, it is still considered a structural isomer because it has the same number of carbon atoms as cyclohexane but instead of forming a ring, they are in a straight chain with a methyl group attached to the second carbon atom.
Constitutional isomers have the same molecular formula but different arrangements of atoms. For instance, the constitutional isomers of cyclohexane derivatives are 1,2-dichlorocyclohexane and 1,4-dichlorocyclohexane. These compounds contain a cyclohexane ring and each have two chlorine atoms. They differ in how the chlorine atoms are bonded to the ring. This type of relationship represents what are called constitutional isomers.
Isomers of a compound that differ only in their three-dimensional (spatial) orientation are called stereoisomers. An example of stereoisomers is cis-1,2-dihydroxycyclohexane and trans-1,2-dihydroxycyclohexane. The two alcohol groups are on the same face of the ring in one case, but on opposite faces in the other. Cyclohexane itself is formed by the hydrogenation of benzene and cyclohexene using a catalyst.
In conclusion, 2-methylhexane is a structural isomer of cyclohexane because it has the same number of carbon atoms but a different structural layout. However, it is not an isomer of cyclohexane in the strictest sense because it does not have the same molecular formula.
Sexual Misconduct with Minors: Indiana's Strict Laws
You may want to see also
Frequently asked questions
A constitutional isomer, also known as a structural isomer, is a compound that has the same molecular formula as another compound but a different arrangement of atoms.
Cyclohexene has six carbon atoms arranged in a ring with one double bond and the molecular formula C6H12. Cyclohexane is not a constitutional isomer of cyclohexene because it has the same molecular formula but lacks the double bond structure. 3-methylpent-2-ene, on the other hand, is a structural isomer of cyclohexane as it has the same molecular formula but a different bonding structure and connectivity.
Other examples of constitutional isomers include 1,2-dichlorocyclohexane and 1,4-dichlorocyclohexane, which are isomers of cyclohexane. In butane (C4H10), n-butane (a straight chain) and isobutane (a branched chain) are constitutional isomers.
Stereoisomers have the same molecular formula and chemical bonds between atoms but differ in their three-dimensional (spatial) orientation of atoms.


































