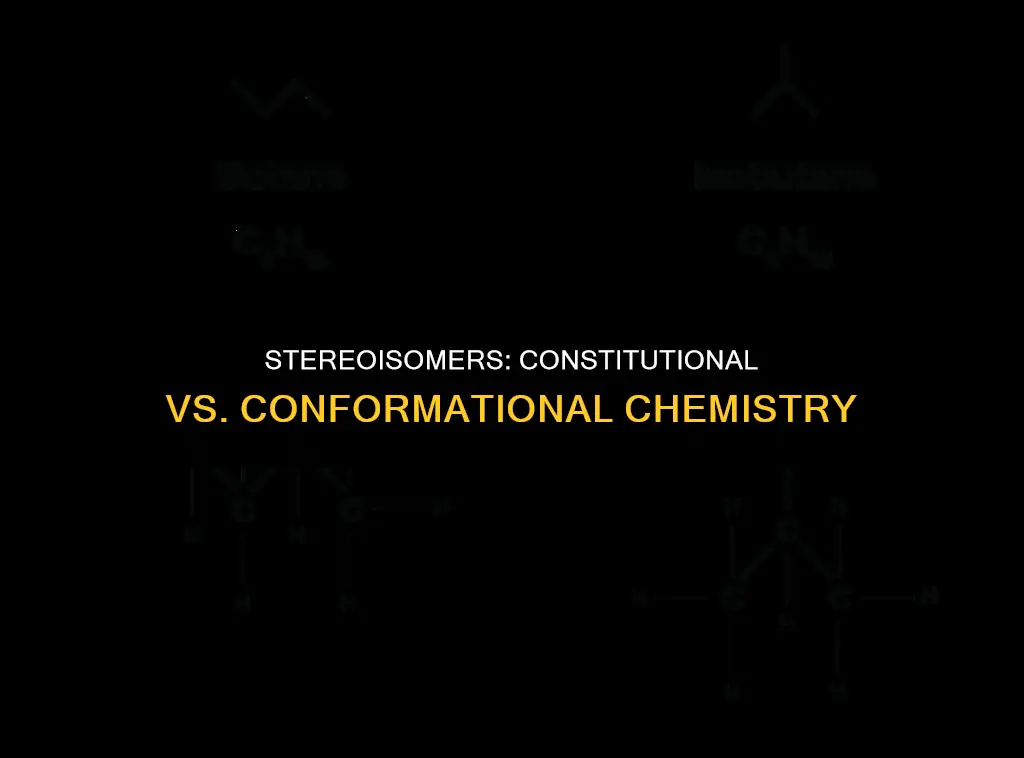
Isomers are compounds with the same molecular formula but different structures. There are two main types of isomers: constitutional isomers and stereoisomers. Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the connectivity of their atoms, resulting in different structures. Stereoisomers, on the other hand, have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms, leading to different physical and chemical properties. Conformers, or conformational isomers, are a subset of stereoisomers, representing molecules that have the same molecular formula and connectivity but differ in the rotation around a single sigma bond, resulting in different spatial arrangements. Understanding the distinctions between constitutional and conformational stereoisomers is crucial for analyzing molecular relationships, particularly in organic chemistry.
| Characteristics | Constitutional Isomers | Stereoisomers |
|---|---|---|
| Molecular Formula | Same | Same |
| Connectivity | Different | Same |
| Number of Molecules | Two or more | Two |
| Types | Structural isomers | Cis-trans isomers, enantiomers, diastereomers |
| Superimposition | Not possible | Possible through rotation of bonds or the molecule itself |
| Conformers | N/A | Considered the same molecule but in different rotational states |
Explore related products
What You'll Learn
- Constitutional isomers have the same molecular formula but differ in connectivity
- Stereoisomers have the same connectivity but different shapes
- Conformers are considered the same molecule but differ in rotational states
- Stereoisomers can be further classified into enantiomers and diastereomers
- Constitutional isomers are also known as structural isomers

Constitutional isomers have the same molecular formula but differ in connectivity
Constitutional isomers and conformational stereoisomers are two different types of isomers. Isomers are compounds that share the same molecular formula but differ in structure or arrangement.
Constitutional isomers, also known as structural isomers, share the same molecular formula but differ in the connectivity of their atoms. This means that the atoms are connected in different ways, leading to different structures. For example, butane (C4H10) and isobutane (C4H10) have the same molecular formula but different structures. Butane has a straight chain, while isobutane has a branched chain. This difference in connectivity classifies them as constitutional isomers.
Conformational isomers, on the other hand, are a type of stereoisomer. Stereoisomers have the same molecular formula and connectivity but differ in their spatial arrangement. This means that they are not the same molecule, as they differ in the arrangement of their atoms in space. Conformational isomers are molecules that differ only by rotation around a single sigma bond. These rotations do not break any bonds and can occur freely, leading to different spatial arrangements. For example, in ethane (C2H6), the rotation around the C-C sigma bond can lead to different conformations such as staggered and eclipsed forms.
It is important to distinguish between constitutional isomers and conformational stereoisomers, especially in organic chemistry, as it helps to understand molecular behaviour and reactivity. While constitutional isomers differ in the connectivity of their atoms, conformational isomers are the same molecule but in different rotational states.
In summary, constitutional isomers have the same molecular formula but differ in the way their atoms are connected, resulting in different structures. Conformational stereoisomers, on the other hand, are the same molecule but differ in the rotation around a single bond, leading to different spatial arrangements.
Theological Virtues: What They Are Not
You may want to see also

Stereoisomers have the same connectivity but different shapes
Stereoisomers and constitutional isomers are two broad categories of isomers—molecules with the same chemical formula. While constitutional isomers differ in the connectivity of their atoms, stereoisomers have the same connectivity but different shapes.
Constitutional isomers, also known as structural isomers, have the same molecular formula but different structures. This means that the atoms within the molecules are connected differently, resulting in distinct shapes. These differences in connectivity and structure lead to variations in the physical and chemical properties of the isomers.
On the other hand, stereoisomers share the same molecular formula and connectivity but differ in their spatial arrangement. Stereoisomers are fundamentally different molecules with fixed shapes. They can be further classified into two types: enantiomers and diastereomers. Enantiomers are stereoisomers that are non-superimposable mirror images of each other. Diastereomers, on the other hand, are stereoisomers that are not mirror images.
Conformational isomers, or conformers, are a subset of stereoisomers. They are considered the same molecule but differ in the rotation about a single bond, resulting in different rotational states. These rotations occur around a sigma bond without breaking any bonds, leading to distinct spatial arrangements. While conformers are generally considered the same molecule, stereoisomers represent distinct molecules due to their differences in spatial arrangement.
In summary, stereoisomers have the same connectivity between their atoms but exhibit different shapes due to variations in the spatial arrangement of those atoms. This distinction between stereoisomers and constitutional isomers is crucial for understanding molecular behaviour and reactivity, especially in the field of organic chemistry.
The Constitution: Guarding Against Tyranny
You may want to see also

Conformers are considered the same molecule but differ in rotational states
Conformers, or conformational isomers, are molecules that have the same molecular formula and connectivity but differ in their rotational states. They are considered the same molecule but differ in the rotation about a single sigma bond. This rotation does not break any bonds and can occur freely, resulting in different spatial arrangements. For example, in ethane (C2H6), the rotation around the C-C sigma bond can lead to different conformations, such as staggered and eclipsed forms.
Conformers are closely related to stereoisomers, which are molecules with the same molecular formula and connectivity but differing in their spatial arrangement. Stereoisomers represent fundamentally different molecules with fixed shapes, whereas conformers are considered the same molecule with different rotational states. This distinction is essential in organic chemistry for understanding molecular behaviour and reactivity.
The concept of conformers is based on the idea that molecules can exist in different rotational states without changing their fundamental structure. These rotational states arise from the rotation of the molecule itself or the rotation of bonds within the molecule (conformational changes). In organic chemistry, two molecules that can be superimposed on each other through these rotations are considered identical copies of the same molecule.
The index of hydrogen deficiency (IHD) is a calculation used to determine the degree of unsaturation in a molecule. It takes into account the number of rings, double bonds, and triple bonds present. For example, benzene (C6H6) has an IHD of 4, indicating three double bonds and one ring. IHD is a useful tool for understanding the rotational states of conformers and their structural variations.
In summary, conformers are considered the same molecule but exhibit different rotational states due to the rotation of the molecule or the rotation of bonds within the molecule. This distinction is important in organic chemistry for understanding molecular relationships and reactivity. By utilizing tools like IHD, scientists can gain insights into the structural variations and behaviour of conformers.
The Constitution's Lines: A Comprehensive Count
You may want to see also
Explore related products

Stereoisomers can be further classified into enantiomers and diastereomers
Stereoisomers are sets of molecules that have the same chemical formula and connectivity but differ in the spatial arrangement of their atoms. They can be further classified into enantiomers and diastereomers.
Enantiomers are stereoisomers that are non-superimposable mirror images of each other. They have the same connectivity but differ in their spatial orientation. They are also referred to as optical isomers because of the way they interact with light. Each molecule in a pair of enantiomers bends polarized light in opposing directions, either clockwise (+) or counterclockwise (-). Enantiomers must have at least one chiral center, which is a central atom connected to four unique atoms or groups of atoms.
Diastereomers, on the other hand, are stereoisomers that are not mirror images of each other. They are also non-superimposable. Like enantiomers, diastereomers have the same connectivity but differ in their spatial arrangement. They share the same configuration on at least one but not all chiral centers. Cis- and trans-dichloroethene are examples of diastereomers.
Determining whether molecules are enantiomers, diastereomers, or the same can be done through building models of the molecules and comparing them. Another method is to identify the chiral centers in each molecule and determine their absolute configuration (R or S).
Wild Animal Sex: R-Rated or Not?
You may want to see also

Constitutional isomers are also known as structural isomers
Constitutional isomers, also known as structural isomers, are a type of isomer that shares the same molecular formula but differs in the connectivity of their atoms. This means that the atoms are connected in different ways, leading to distinct structures. For example, butane (C4H10) and isobutane (C4H10) share the same molecular formula but differ in structure. Butane has a straight chain, while isobutane has a branched chain. This difference in connectivity classifies them as constitutional isomers.
Conformational isomers, on the other hand, are considered a subset of stereoisomers. They are essentially the same molecule but differ in the rotation about a single bond. These rotations do not break any bonds and can occur freely, resulting in different spatial arrangements. For instance, in ethane (C2H6), the rotation around the C-C sigma bond can lead to staggered and eclipsed forms.
Stereoisomers, including conformational isomers, represent fundamentally distinct molecules with fixed shapes. While constitutional isomers differ in the connectivity of their atoms, stereoisomers share the same connectivity but differ in their spatial arrangement. This distinction is crucial for understanding molecular behaviour and reactivity in organic chemistry.
It is important to note that a pair of isomers can be either constitutional isomers or stereoisomers, but not both simultaneously. Constitutional isomers are identified by ensuring that the molecules share the same molecular formula and then checking the connectivity of their atoms. If the atoms are connected differently, they are classified as constitutional isomers.
In summary, constitutional isomers, or structural isomers, differ in the way atoms are connected, resulting in distinct structures, while conformational isomers are a type of stereoisomer that differs only in the rotation about a single bond, leading to different spatial arrangements of the same molecule.
Constitutional vs Legislative Courts: Understanding the Key Differences
You may want to see also
Frequently asked questions
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but a different structure and connectivity of their atoms. Stereoisomers are a broader category of isomers that encompass constitutional isomers. Conformational isomers, or conformers, are a type of stereoisomer and are considered the same molecule with different rotational states.
Constitutional isomers differ in the connectivity of their atoms, resulting in different structures. Conformational isomers, on the other hand, have the same molecular formula and connectivity but differ in their rotational states around a single bond, leading to different spatial arrangements.
Butane (C4H10) and isobutane (C4H10) are constitutional isomers. Butane has a straight-chain structure, while isobutane has a branched chain, making them different in terms of connectivity and structure. An example of conformational isomers is ethane (C2H6), where rotation around the C-C sigma bond results in different conformations such as staggered and eclipsed forms.