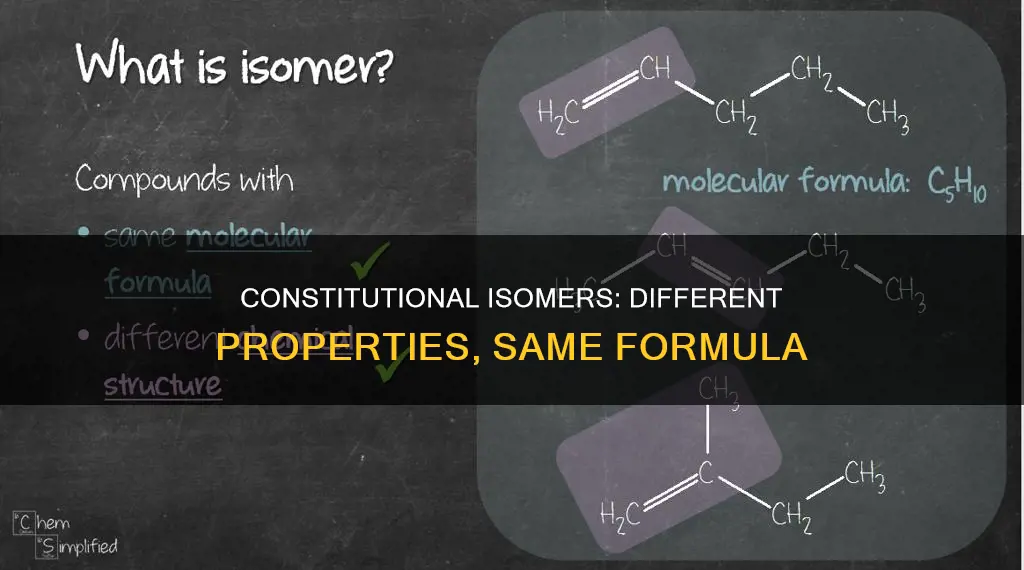
Constitutional isomers are compounds with the same molecular formula but different connectivities. They are also known as structural isomers. The connectivity of atoms is crucial in defining the properties of a molecule. For example, ethanol (ethyl alcohol) and dimethyl ether have the same molecular formula, but different connections between their atoms. This means that they are different molecules with different properties. Constitutional isomers can have the same or different functional groups, but they are located at different points on the carbon skeleton. The number of possible constitutional isomers increases with the number of atoms in the molecule.
| Characteristics | Values |
|---|---|
| Molecular formula | Same |
| Connectivity | Different |
| Physical properties | Different |
| Chemical properties | Different |
| Number of atoms | Same |
| Functional groups | Same or different |
Explore related products
What You'll Learn

Constitutional isomers have the same molecular formula
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different connectivities. This means that the atoms are linked in different ways, resulting in different structures. For example, ethanol (or ethyl alcohol) and dimethyl ether are constitutional isomers of each other, with the molecular formula C2H6O. However, their functional groups differ: ethyl alcohol has an atomic connectivity of C-C-O, while the C-O-C connectivity in dimethyl ether forms an ether.
The number of possible constitutional isomers increases exponentially with the number of atoms in a molecule. For instance, while there is only one possible isomer for CH4 (methane), there are 355 possible isomers for dodecane (C12H26). Despite sharing the same molecular formula, isomers may exhibit very different physical and chemical properties, such as boiling point, melting point, and chemical reactivity. For example, cyclohexane (b.p. 63 °C) and 1-hexene (80 °C) both have the molecular formula C6H12 but differ in their physical properties.
The unique connectivity of constitutional isomers leads to different structural forms and properties, highlighting the importance of both molecular composition and structural arrangement in organic chemistry. The connectivity of atoms is crucial in defining the properties of a molecule. For instance, butane and isobutane, both with the molecular formula C4H10, differ in their carbon backbones. Butane has an uninterrupted chain of carbon atoms, while isobutane has only three carbon atoms connected in sequence.
To identify constitutional isomers, one must first ensure that the molecules have the same molecular formula. The second step is to check the connectivity of the atoms. If the atoms are connected differently, the molecules are considered constitutional isomers. The Hydrogen Deficiency Index (HDI) is a useful tool for determining constitutional isomers, as it helps identify the presence of double or triple bonds in a molecule. By knowing the HDI for a molecule, one can draw various constitutional isomers with the correct structural motifs.
In summary, constitutional isomers share the same molecular formula but differ in the connectivity of their atoms, leading to distinct structures and properties. This understanding of isomerism is crucial in fields like organic chemistry, where the relationship between similar compounds and their unique properties is of significant importance.
Exploring Democracy: Gordon Wood's Constitutional Insights
You may want to see also

They differ in their atomic connectivity
Isomers are molecules that share the same molecular formula but differ in the way their atoms are arranged. They can have different connectivities, rotations, or spatial arrangements and sometimes exhibit different chemical or physical properties.
Constitutional isomers, also known as structural isomers, have the same molecular formula but different connectivities. They are molecules with the same atoms connected differently. For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers. They have the same atoms in the same ratios, but the connections between those atoms are different. The atomic connectivity is C—C—O in ethyl alcohol, and the oxygen atom is part of an alcohol. In contrast, the C—O—C connectivity in the isomer forms an ether.
Constitutional isomers can also have different functional groups. For example, both ethyl alcohol and dimethyl ether have the same molecular formula: C2H6O. However, their functional groups differ. Constitutional isomers can also have the same functional groups, but these groups are located at different points on the carbon skeleton. For instance, the isomers 1-propanol and 2-propanol have a hydroxyl group on different carbon atoms.
Constitutional isomers can differ in their carbon backbones. For example, butane and isobutane are constitutional isomers of C4H10. Butane has an uninterrupted chain of carbon atoms, while isobutane has only three carbon atoms connected in sequence. The number of possible constitutional isomers increases exponentially with the number of atoms in the molecule. For instance, while there is only one possible isomer for CH4 (methane), there are 355 possible isomers for dodecane (C12H26).
Constitutional isomers are distinct from stereoisomers, which have the same connectivity but differ in the arrangement of their atoms in space. Stereoisomers can be further divided into configurational isomers (enantiomers and diastereomers) and conformational isomers. Enantiomers are chiral molecules that are non-superimposable mirror images of each other. They have the same connectivity and physical properties but differ in their optical properties. Diastereomers are not mirror images of each other and can have different physical properties and chemical reactivities. They arise when a molecule has two or more chiral centres, leading to various possible spatial arrangements.
Founding Fathers: Slave Owners and the Constitution
You may want to see also

They have different structural forms
Constitutional isomers are compounds that have the same molecular formula but differ in their atomic connectivity, resulting in different structural forms and properties. These structural differences can be subtle, but they can profoundly affect the physical and chemical properties of isomers.
For example, ethanol (or ethyl alcohol) and dimethyl ether are constitutional isomers with the same molecular formula, C2H6O. However, their atomic connectivity differs. In ethanol, the atomic connectivity is C-C-O, with the oxygen atom being part of an alcohol. On the other hand, dimethyl ether has a C-O-C connectivity, forming an ether. These differences in connectivity lead to distinct structural forms, making them unique molecules with different properties.
Another example is the pair of isomers, 1-propanol and 2-propanol. Both isomers have a hydroxyl group, but this group is located on different carbon atoms in each isomer, resulting in different structural arrangements.
The number of possible constitutional isomers increases exponentially with the number of atoms in a molecule. For instance, while there is only one possible isomer for methane (CH4) and ethane (C2H6), the number of possible isomers for dodecane (C12H26) jumps to 355. This highlights the importance of understanding both molecular composition and structural arrangement in organic chemistry.
Constitutional isomers can also differ in their carbon backbones. Butane (C4H10) and isobutane (C4H10) provide an illustration of this. Butane has an uninterrupted chain of carbon atoms, while isobutane has only three carbon atoms connected in sequence. This variation in carbon backbone contributes to their distinct structural forms.
Attorney Appearance in Maryland: What Constitutes It?
You may want to see also
Explore related products

They have different physical and chemical properties
Constitutional isomers are compounds with the same molecular formula but different connectivities. This means that the atoms are connected in different ways, leading to different structures and, consequently, different physical and chemical properties.
For example, ethanol (or ethyl alcohol) and dimethyl ether are constitutional isomers with the same molecular formula, C2H6O. However, their atomic connectivity differs. In ethanol, the atomic connectivity is C–C–O, with the oxygen atom being part of an alcohol. On the other hand, dimethyl ether has a C–O–C connectivity, forming an ether. These differences in connectivity result in distinct physical and chemical properties between the two compounds.
Another example of constitutional isomers is butane (C4H10) and isobutane (C4H10). Butane has an uninterrupted chain of carbon atoms, while isobutane has only three carbon atoms connected in sequence. This variation in carbon backbone structure leads to different physical and chemical characteristics.
The number of possible constitutional isomers increases exponentially with the number of atoms in a molecule. For instance, while methane (CH4) and ethane (C2H6) have only one possible isomer, dodecane (C12H26) can have up to 355 isomers. The ability to form various isomers by rearranging carbon atoms is particularly notable in organic chemistry, leading to the synthesis of new molecules with unique properties.
Constitutional isomers can also have different functional groups or the same functional groups located at different points on the carbon skeleton. For instance, ethyl alcohol and dimethyl ether have different functional groups, with ethanol having an alcohol group and dimethyl ether having an ether group. Meanwhile, isomers like 1-propanol and 2-propanol share a hydroxyl group but have it located on different carbon atoms. These differences in functional groups and their positions further contribute to the diverse physical and chemical properties of constitutional isomers.
Understanding Parental Manipulation: New Jersey's Legal Standpoint
You may want to see also

They can have different functional groups
Constitutional isomers are substances that have the same molecular formula but differ in their connectivity. For example, butane and isobutane are constitutional isomers of C4H10. Butane has an uninterrupted chain of carbon atoms, while isobutane only has three carbon atoms connected in sequence.
Constitutional isomers can have different functional groups. A functional group is a group of atoms in a molecule with distinctive chemical properties, regardless of the other atoms in the molecule. The same functional group will undergo the same or similar chemical reactions regardless of the rest of the molecule's composition. This enables systematic prediction of chemical reactions and behavior of chemical compounds and the design of chemical synthesis.
For example, ethyl alcohol and dimethyl ether are constitutional isomers with the same molecular formula: C2H6O. However, their functional groups differ. The atomic connectivity in ethyl alcohol is C—C—O, and the oxygen atom is part of an alcohol. On the other hand, the C—O—C connectivity in dimethyl ether forms an ether.
Constitutional isomers can also have the same functional groups but located at different points on the carbon skeleton. For instance, the isomers 1-propanol and 2-propanol have a hydroxyl group on different carbon atoms.
The number of possible constitutional isomers increases exponentially with the number of atoms in the molecule. For example, while there is only one possible isomer for CH4 (methane), there are 355 possible isomers for dodecane (C12H26).
Constitution and Expats: Who Does It Cover?
You may want to see also
Frequently asked questions
Constitutional isomers are compounds with the same molecular formula but different connectivities.
Constitutional isomers have the same number of atoms and the same degree of HDI (Index of Hydrogen Deficiency).
Constitutional isomers can have different structures, different physical properties, and different chemical properties.