Isomers are compounds that share the same molecular formula but differ in structure or arrangement. They can be classified into different types based on their molecular formula, connectivity, and shape. Constitutional isomers, also known as structural isomers, are a specific type of isomer that share the same molecular formula but differ in atomic connectivity and bonding patterns. They can have different functional groups or the same functional groups located at different points on the carbon skeleton. For example, ethanol and dimethyl ether are constitutional isomers with the same molecular formula (C2H6O) but different atomic connectivities. Understanding the relationship between isomers is crucial in chemistry, especially in organic chemistry, where concepts like the Index of Hydrogen Deficiency (IHD) help determine saturation and structural variations.
| Characteristics | Values |
|---|---|
| Definition | Compounds that have the same chemical formula but differ in the way in which the constituent atoms are connected to one another |
| Other names | Structural isomers |
| Identification | Counting the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index) |
| Examples | Butane and isobutane, both having the molecular formula C4H10 but different structural arrangements |
Explore related products
What You'll Learn
- Constitutional isomers have the same molecular formula but different connectivity of atoms
- Stereoisomers have the same connectivity but differ in the arrangement of atoms in space
- Positional isomers are a type of constitutional isomer
- Constitutional isomers can be differentiated using gas chromatography and mass spectrometry
- Constitutional isomers can be identified by counting the number of carbons and the degree of unsaturation

Constitutional isomers have the same molecular formula but different connectivity of atoms
To identify the relationship between compounds, it is necessary to analyse their molecular formulas and structures. This helps to explain the variety of chemical compounds and their behaviours. Compounds with the same molecular formula but different structures are called isomers.
Constitutional isomers, also known as structural isomers, are a type of isomer. They have the same molecular formula but differ in the way their atoms are connected. In other words, they have different structural arrangements. For example, butane (C₄H₁₀) has a straight-chain structure, whereas isobutane is branched. This difference in arrangement leads to distinct chemical behaviours.
The connectivity of atoms is crucial in defining the properties of a molecule. For instance, ethanol and dimethyl ether both have the formula C2H6O and the same molecular mass, but they have completely different physical and chemical properties.
To determine whether two molecules are constitutional isomers, one can count the number of carbons and the degree of unsaturation (Hydrogen Deficiency Index). If all the atoms are the same and the molecules have the same HDI, they are likely constitutional isomers. However, for larger molecules, it may be necessary to name the molecules according to IUPAC nomenclature rules.
Sugar Conversions: Tablespoons to Grams
You may want to see also

Stereoisomers have the same connectivity but differ in the arrangement of atoms in space
Stereoisomers are a type of isomer that has the same connectivity of atoms but differs in the spatial arrangement of atoms. This means that stereoisomers have the same molecular formula and the same number of atoms, but the atoms are arranged differently in space. For example, trans-1,2-dibromocyclobutane and cis-1,2-dibromocyclobutane are stereoisomers of each other because they have the same molecular formula and connectivity, but the bromine atoms are positioned differently in space. In trans-1,2-dibromocyclobutane, the bromine atoms are on opposite sides of the cyclobutane ring, while in cis-1,2-dibromocyclobutane, the bromine atoms are on the same side of the ring.
Stereoisomers can be further classified into two types: enantiomers and diastereomers. Enantiomers are stereoisomers that are mirror images of each other but are not superimposable. This means that they have the same molecular formula and connectivity, but their atoms are arranged differently in space, such that one isomer is a reflection of the other. Enantiomers have identical physical properties, except for optical rotation, which means they rotate light in opposite directions. Diastereomers, on the other hand, are stereoisomers that are not mirror images of each other. In a pair of diastereomers, at least one but not all of the chiral centers are opposite.
The concept of stereoisomers is important in organic chemistry because it helps to explain the physical and theoretical reasons behind the formation and structures of many organic molecules. The energy embedded in these molecules can be significantly affected by changes in atomic placement, so understanding the spatial arrangement of atoms is crucial.
It is important to distinguish between stereoisomers and constitutional isomers. Constitutional isomers have the same molecular formula but differ in their atomic connectivity. This means that the atoms are connected differently, resulting in different structures and properties. For example, butane (C4H10) can have two structures that satisfy its chemical formula: n-butane and isobutane. These two compounds are constitutional isomers because they have the same molecular formula but different connectivities, leading to distinct physical and chemical properties.
When Emergencies Warrant Imaging Scans
You may want to see also

Positional isomers are a type of constitutional isomer
Constitutional isomers are compounds that have the same chemical formula but differ in the way their atoms are bonded or connected. For example, butane (C4H10) can have several structures that satisfy its chemical formula. While they have the same number of carbons and hydrogens, they are connected differently. These compounds are said to be constitutional or structural isomers. Another example is the formula C2H6O, which could represent ethanol (drinking alcohol) or dimethyl ether. Despite having the same molecular mass, these two compounds have distinct physical and chemical properties. This highlights the significance of the connectivity of atoms in defining the characteristics of a molecule.
Now, let's focus on positional isomers, which are indeed a type of constitutional isomer. Positional isomers, also known as regioisomers, are structural isomers that differ only in the position of a functional group, substituent, or some other feature on the same "parent" structure. In simpler terms, they have the same chemical formula and bonding patterns, but the location of specific atoms or groups within the molecule varies.
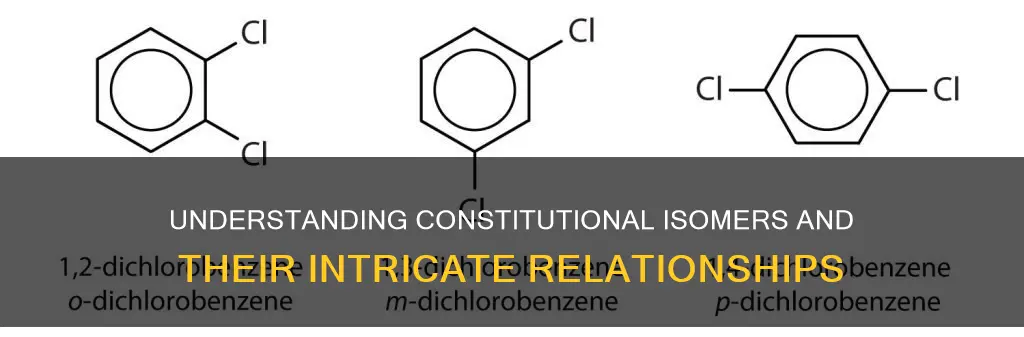
To illustrate the concept of positional isomers, let's consider the example of chlorobenzene. When one of the hydrogen atoms in benzene is replaced by chlorine, the resulting compound is chlorobenzene. However, this substitution reduces the number of possible positional isomers. Initially, the six hydrogens in benzene are structurally equivalent, meaning they can be interchanged without altering the molecule's structure. But once the chlorine atom is introduced, only one permutation remains viable, corresponding to flipping the molecule while keeping the chlorine fixed. This results in three different equivalence classes for the remaining hydrogens: ortho, meta, and para. Consequently, a second substitution of hydrogen by chlorine can yield three positional isomers: 1,2- or ortho-, 1,3- or meta-, and 1,4- or para-dichlorobenzene.
The concept of positional isomers is also applicable to other compounds, such as ethanol and propanol. In the case of ethanol (C2H5OH), there is only one structural isomer due to the structural equivalence of the six hydrogens in ethane (C2H6). On the other hand, propanol exhibits two positional isomers (1-propanol and 2-propanol) because the eight hydrogens in propane (C3H8) can be divided into two structural equivalence classes. Similarly, butanol has two positional isomers, while pentanol and hexanol have three.
In summary, positional isomers are a specific type of constitutional isomer, where the compounds have the same chemical formula and bonding patterns but differ in the placement of certain atoms or groups within the molecule. This distinction in the arrangement of atoms leads to variations in the properties of the resulting compounds, showcasing the importance of structural isomerism in chemistry.
Understanding Adultery: UK Law and Affairs Explained
You may want to see also
Explore related products
$5.28 $5.98

Constitutional isomers can be differentiated using gas chromatography and mass spectrometry
Constitutional isomers have the same chemical formula but differ in the way their atoms are connected. For instance, butane (C4H10) can have several structures that satisfy its chemical formula; these are said to be constitutional or structural isomers.
Differentiating structural isomers using gas chromatography (GC) and mass spectrometry (MS) can be challenging, and further structural elucidation is often required. However, it is possible to differentiate constitutional isomers using these techniques.
One method is to use gas chromatography coupled with vacuum ultraviolet ionization mass spectrometry (GC-VUV-MS). This technique has been used to study the constitutional isomers present in complex hydrocarbon mixtures, such as lubricating oil, diesel fuel, and crude oil. By varying the EI (electron impact) ionization in mass spectrometry, the identification of individual isomers and homologous compound groups can be significantly enhanced. High-resolution mass spectrometry also facilitates the grouping of observed chromatographic peaks by their Kendrick mass defect, providing a mechanism to identify homologous series.
Another approach is to employ comprehensive 2D gas chromatography time-of-flight mass spectrometry (GC x GC-ToF-MS). This technique is powerful in separating and identifying numerous compounds within complicated hydrocarbon mixtures.
Additionally, structural isomer-specific ions can be used to discriminate between isomers. While the fragmentation pattern of structural isomers may be nearly identical, there are specific ions that can be identified through MS/MS analysis. Ion mobility technology further separates ions derived from structural isomers based on their size, shape, and collisional cross-section, providing a structure-specific value.
Understanding Treason and Its Punishment in the Constitution
You may want to see also

Constitutional isomers can be identified by counting the number of carbons and the degree of unsaturation
Constitutional isomers are compounds with the same molecular formula but a different connectivity of atoms. They can have the same or different functional groups. To identify constitutional isomers, one can start by counting the number of carbon atoms and calculating the degree of unsaturation, often represented as the Index of Hydrogen Deficiency (IHD). The IHD is a useful tool for determining the degree of unsaturation in a molecule, where a double bond or ring contributes 1 to the IHD, and a triple bond contributes 2. This process of analysing molecular structures through atom counting and IHD calculation is particularly beneficial for more complex molecules.
For example, two molecules with the same composition of 5 carbon atoms, 1 oxygen atom, and 1 fluorine atom may be constitutional isomers. The first molecule has an IHD of 1, indicating the presence of a double bond or ring. In contrast, the second molecule has an IHD of 0, signifying no double bonds or rings are present. Thus, they are constitutional isomers with the same formula but different connectivity.
Another example is the formula C2H6O, which can represent ethanol (drinking alcohol) or dimethyl ether. Despite having the same molecular mass, these two molecules have different physical and chemical properties due to their different atomic connectivity.
Additionally, constitutional isomers can be identified by checking the number of carbons and heteroatoms and comparing the degree of unsaturation. For instance, molecules A, B, and D are constitutional isomers because they have the same number of identical atoms and the same degree of HDI, i.e., no ring or multiple bonds. However, molecule F is the same as molecule A but flipped by 180 degrees.
Delta's Frequent Flyer: What Makes You One?
You may want to see also
Frequently asked questions
Constitutional isomers share the same molecular formula but differ in their connectivity, i.e., the way in which the constituent atoms are connected to one another. Stereoisomers, on the other hand, have the same connectivity but differ in the arrangement of atoms in space.
Examples of constitutional isomers include ethanol (ethyl alcohol) and dimethyl ether, which have the same molecular formula (C2H6O) but different atomic connectivity. Other examples include butane and isobutane, which differ in their carbon backbones.
To identify constitutional isomers, we first look at the molecular formula. From there, we can calculate the Index of Hydrogen Deficiency (IHD) to understand the structural features of the molecule, such as the presence of rings, double bonds, or triple bonds. By knowing the HDI, we can draw various constitutional isomers with the correct structural motifs.











































