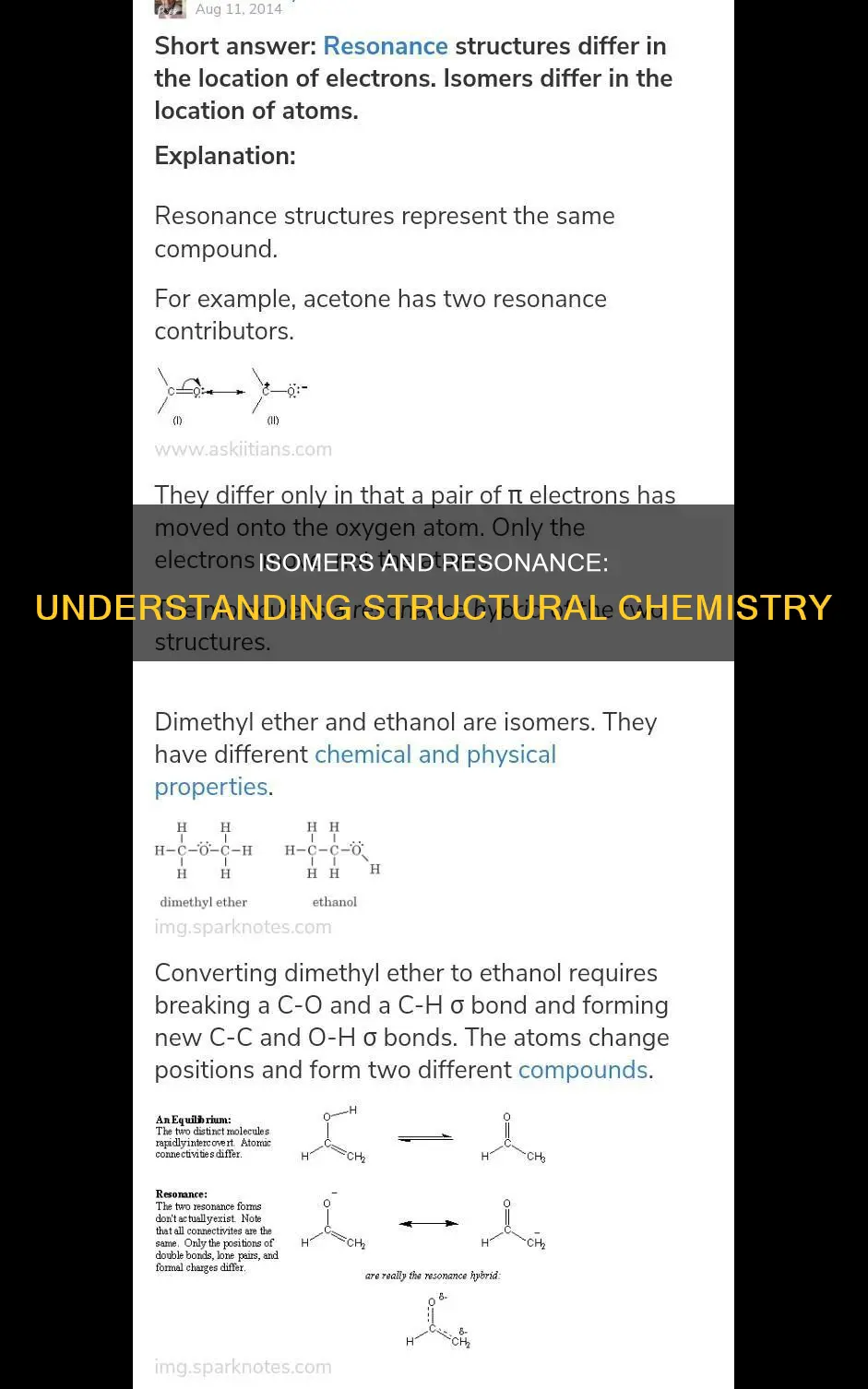
Isomerism and resonance structures are two fundamental concepts in chemistry that describe the arrangement of atoms and molecules. Isomerism refers to the phenomenon where molecules have the same number of atoms and the same formula but differ in their atomic arrangement, resulting in distinct chemical and physical properties. There are several types of isomers, including constitutional isomers, which have the same formula but different connectivity, and stereoisomers, which have the same connectivity but differ in the spatial arrangement of their atoms. On the other hand, resonance structures depict molecules with the same atoms and arrangement, but the electrons can move freely, distributing the charge. While isomers represent distinct compounds with different properties, resonance structures are fictitious representations of the same compound, illustrating the movement of electrons and providing insight into its stability. Understanding these concepts is crucial in fields like organic chemistry, enabling chemists to predict and explain the behaviour of various chemical species.
| Characteristics | Constitutional Isomers |
|---|---|
| Definition | Isomerism refers to the presence of molecules with the same number of the same type of atoms but with distinct chemical and physical properties. |
| Formula | Constitutional isomers have the same formula but different connectivity. |
| Types | There are two types of isomers: positional isomers and functional isomers. |
| Comparison | A molecule can be a constitutional isomer, diastereomer, enantiomer, or none, all at the same time, depending on which molecule you are comparing it to. |
| Characteristics | Resonance Structures |
| --- | --- |
| Definition | Resonance structures have the same atoms in the same arrangement, but the electrons can easily move around the molecule, spreading the charge around. |
| Formula | The molecular formulas for resonance structures are the same. |
| Lewis Structure | The Lewis structure symbolizes each atom and its position in the molecular structure. |
| Comparison | Resonant structures aren't in sync with one another. |
Explore related products
What You'll Learn
- Constitutional isomers have the same formula but different connectivity
- Resonance structures are fictitious, whereas isomers are real
- Isomers have different arrangements of atoms and electrons
- Resonance structures can be used to depict the movement of electrons
- Isomerism refers to molecules with the same atoms but distinct properties

Constitutional isomers have the same formula but different connectivity
Isomerism refers to the presence of molecules with the same number of the same type of atoms, and thus the same molecular formula, but with distinct chemical and physical properties. In other words, isomers are chemical molecules that have the same components but are not identical. There are two types of isomers: positional isomers and functional isomers.
Constitutional isomers are a type of isomer that have the same molecular formula but different connectivity. This means that constitutional isomers have the same types and numbers of atoms but differ in the way these atoms are bonded or connected to each other. For example, two bracelets made of five red and five green beads can be configured in various ways depending on the order of the colours. Each bracelet has the same components but a different arrangement of the beads.
Another way to think about constitutional isomers is through the concept of "brother" and "sister". Just as a person can be a daughter to their mother and a sister to their brother, a molecule can be a constitutional isomer to one molecule and a stereoisomer to another. The relationship between molecules depends on the context of the comparison.
It is important to distinguish constitutional isomers from resonance structures. While both concepts involve the arrangement of atoms, they differ in their focus. Constitutional isomers refer to distinct molecules with the same formula but different connectivity, whereas resonance structures describe a single molecule with multiple possible arrangements of electrons. In other words, resonance structures depict the movement of electrons within a molecule, resulting in different resonance forms or contributors that help explain the molecule's stability and properties.
Alien and Sedition Acts: Constitutional or Not?
You may want to see also

Resonance structures are fictitious, whereas isomers are real
Isomerism and resonance structures are two distinct concepts in chemistry, with key differences in their nature and representation of molecules. Isomerism refers to the presence of molecules with the same number of atoms and the same formula but different arrangements of atoms and electrons, resulting in distinct chemical and physical properties. On the other hand, resonance structures depict the same atoms in the same arrangement but allow for the movement of electrons, spreading the charge around the molecule.
Resonance structures are considered fictitious because they are not real representations of a molecule's structure. Instead, they are used to illustrate the concept of resonance, where electrons can move between different positions in a molecule. This movement of electrons is represented by curved arrow notation in resonance structures. The first resonant structure is not real; it merely indicates the potential for different structures to be connected. The actual molecular structure lies somewhere between these resonant forms.
In contrast, isomers are real and exist as separate compounds. They are not fictitious but rather represent molecules with the same atoms arranged differently. Isomerization refers to the possibility that different compounds can have the same atoms but vary in their arrangement, resulting in distinct chemical and physical properties. There are two main types of isomers: positional isomers and functional isomers. Positional isomers differ in the positions of their functional groups, while functional isomers have different functional groups altogether.
The distinction between resonance structures and isomers lies primarily in their representation of molecular structure. Resonance structures focus on the movement of electrons within a molecule, depicting different electron arrangements that are interconnected. On the other hand, isomers emphasize the arrangement of atoms and functional groups, resulting in unique molecular configurations. Isomers are not merely theoretical constructs but exist as distinct compounds with their own chemical and physical characteristics.
While resonance structures help visualize the concept of resonance and electron movement within a molecule, isomers represent actual variations in molecular structure. Isomers are real and tangible, with different properties that can be observed and measured. They are not interchangeable with resonance structures, as the latter are hypothetical representations used to understand the underlying principles of resonance and electron behaviour in a molecule.
Lincoln's Hat: A Constitution Companion?
You may want to see also

Isomers have different arrangements of atoms and electrons
Isomerism and resonance structures are two distinct concepts in chemistry, with key differences in the arrangement of atoms and electrons.
Isomers are chemical molecules that have the same components but differ in their arrangement. This concept is reflected in the term's etymology, derived from the Greek "isos" (equal) and "meros" (portions). Isomers can be further classified into types such as constitutional isomers, stereoisomers, enantiomers, and diastereomers. Constitutional isomers, also known as structural isomers, possess the same molecular formula but differ in the connectivity of atoms and their arrangement in space. Stereoisomers, on the other hand, have the same connectivity but differ in their spatial arrangement. Enantiomers and diastereomers are subtypes of stereoisomers that are mirror images of each other, with enantiomers being non-superimposable and having opposite effects on polarised light.
Resonance structures, on the other hand, refer to the phenomenon where molecules have the same atoms in the same arrangement, but the electrons can move around the molecule, resulting in different resonance modes. This movement of electrons is often depicted using curved arrow notation. While resonance structures provide insight into the stability and properties of molecules, they are considered fictitious structures that cannot be separated or characterised independently.
The distinction between isomers and resonance structures lies primarily in their atomic and electronic arrangements. Isomers encompass a broad category of molecules that share the same atomic composition but differ in their arrangement, whether in connectivity or spatial configuration. In contrast, resonance structures pertain specifically to the distribution of electrons within a molecule, influencing its bonding and overall stability.
Furthermore, isomers can exhibit distinct chemical and physical properties, despite having the same types and numbers of atoms. This diversity arises from the unique arrangements of atoms within isomers, leading to variations in their molecular shapes, polarities, and interactions with other substances. Consequently, different isomers of the same compound may exhibit different solubilities, melting points, and biological activities.
In summary, isomers and resonance structures represent different concepts in chemistry. Isomers encompass a range of molecules with identical atomic compositions but varying arrangements, resulting in distinct properties. Resonance structures, on the other hand, describe the dynamic distribution of electrons within a molecule, influencing its stability and characteristics without altering its fundamental atomic structure. Understanding these concepts is crucial in fields such as organic chemistry, where the behaviour and reactivity of different molecular forms play a central role.
Exploring the National Constitution Center: A Half-Day Visit
You may want to see also
Explore related products

Resonance structures can be used to depict the movement of electrons
Resonance structures are a set of two or more Lewis structures that collectively describe the electronic bonding of a single polyatomic species, including fractional bonds and fractional charges. They are used when there is more than one way to place double bonds and lone pairs on atoms.
Resonance structures are a way of depicting the movement of electrons, specifically delocalized pi-bonding, within certain molecules or polyatomic ions. This is achieved through curved arrow notation, where the tail of the arrow begins at the electron source and the head points to where the electron will be. The arrows indicate the 'movement' of two pi electrons, although this 'movement' is only notional, as it occurs only in our attempt to visualize delocalized pi bonds.
When drawing resonance structures, there are several rules that must be followed. Firstly, only electrons can move, and the nuclei of the atoms never move. Only pi electrons, single unpaired electrons, and lone pair electrons can move. The total number of electrons in the molecule does not change, and neither do the number of paired and unpaired electrons. When electrons can be moved in more than one direction, they are moved towards the more electronegative atom.
It is important to note that when drawing multiple resonance structures, we are not depicting different molecules, but simply different representations of the exact same molecule. The double-headed arrow between resonance structures indicates that the actual electronic structure is an average of those shown, not that the molecule oscillates between them.
Methods of Constitutional Review: Exploring Different Approaches
You may want to see also

Isomerism refers to molecules with the same atoms but distinct properties
Isomerism and resonance structures are related concepts in chemistry, both dealing with the arrangement of atoms and electrons within molecules. Isomerism refers to the phenomenon where molecules with the same types and numbers of atoms (i.e., the same chemical formula) exhibit distinct chemical and physical properties. In other words, isomers are molecules with identical components but different structures, like rearranging the same set of building blocks to create unique shapes.
There are two main types of isomers: positional isomers and functional isomers, or constitutional isomers and stereoisomers. Constitutional isomers have the same molecular formula but differ in the connectivity of atoms within the molecule. They have different bond connections, resulting in distinct arrangements of atoms in space. Stereoisomers, on the other hand, have the same connectivity of atoms but differ in their spatial arrangement.
Enantiomers and diastereomers are types of stereoisomers. Enantiomers are like non-superimposable mirror images of each other, and they rotate the plane of polarized light in equal but opposite directions. Diastereomers are also stereoisomers, but they are not mirror images of each other.
Resonance structures, on the other hand, describe the ability of electrons to move around a molecule, changing their distribution without altering the fundamental arrangement of atoms. This electron movement, or resonance, results in multiple valid structures for a single molecule, each contributing to the overall description of the molecule's electronic structure. While resonance structures depict different electron arrangements, the underlying atoms and their connections remain unchanged.
In summary, isomerism focuses on molecules with the same atoms arranged differently, leading to distinct properties, while resonance structures illustrate the dynamic nature of electrons within a molecule, providing multiple representations of the same underlying atomic arrangement.
Border Crossing Card: Legal Entry or Not?
You may want to see also
Frequently asked questions
Constitutional isomers have the same formula but different connectivity.
Resonance structures have the same atoms in the same arrangement, but the electrons can move around the molecule, spreading the charge.
Constitutional isomers have different connectivities and arrangements of atoms in space. Resonance structures, on the other hand, only differ in the arrangement of electrons.











































