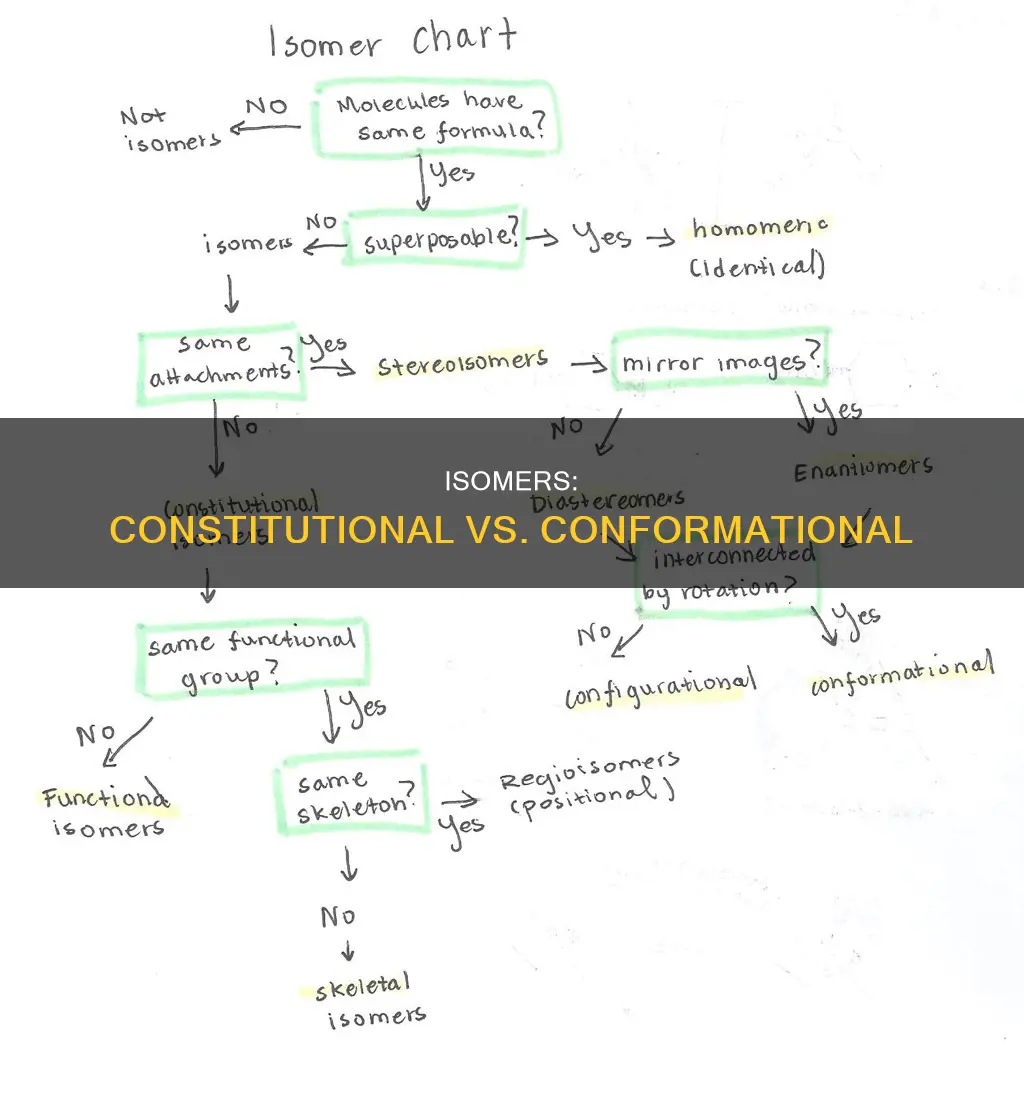
Isomers are compounds with the same molecular formula but different structures or arrangements. There are two main types of isomers: constitutional isomers and conformational isomers. Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of atoms within the molecule. This means the atoms are connected in different ways, leading to different structural formulas. On the other hand, conformational isomers have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds.
| Characteristics | Constitutional Isomers | Conformational Isomers |
|---|---|---|
| Molecular Formula | Same | Different |
| Connectivity | Different | Same |
| Bonds | Different | Same |
| Orientation | Same | Different |
Explore related products
$12.01 $16.97
What You'll Learn

Constitutional isomers have the same molecular formula
Constitutional isomers, also known as structural isomers, share the same molecular formula but have different structural formulas due to the difference in the connectivity of atoms within the molecule. This means that the atoms are the same, but the bonds between them are different, leading to different structures or arrangements. For example, cyclohexane and methylcyclopentane are constitutional isomers as they have the same chemical formula but different bonds between the atoms.
The different connectivity in constitutional isomers can be further subdivided into positional and functional group isomers. Positional isomers have the same connectivity, except for the position of one functional group, which varies. Functional group isomers, on the other hand, have different functional groups, which usually leads to different chemical or physical properties.
Constitutional isomers are different from stereoisomers, which have the same connectivity between atoms but differ in the spatial orientation of those atoms. Stereoisomers can be further divided into configurational isomers (enantiomers and diastereomers) and conformational isomers.
Configurational isomers have the same molecular formula and the same types of bonds but differ in the orientation of the atoms in space. They can be further divided into enantiomers and diastereomers. Enantiomers are chiral molecules that are non-superimposable mirror images of each other. They have the same connectivity and physical properties but differ in their optical properties. Diastereomers, on the other hand, are not mirror images of each other and can have different physical properties and chemical reactivities.
Conformational isomers, or conformers, have the same molecular formula and connectivity between atoms but differ in their spatial arrangement due to the rotation of single bonds. Unlike configurational isomers, which have a fixed spatial arrangement, conformational isomers can interconvert through bond rotation and tend to have very similar energies.
The Constitution's Benefits: A Foundation for Freedom
You may want to see also

Conformational isomers have a different molecular formula
Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of atoms within the molecule. This means that the atoms are connected in different ways, leading to different structural formulas. For example, cyclohexane and methylcyclopentane are constitutional isomers.
Conformational isomers, on the other hand, have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. These isomers can be thought of as different forms of the same molecule that can interconvert by rotation around single bonds. For instance, butane's conformational isomers include staggered and eclipsed forms.
The key distinction between constitutional and conformational isomers lies in their connectivity and spatial arrangement. Constitutional isomers have different connectivities, meaning the atoms are bonded or connected differently. In contrast, conformational isomers have the same connectivity but differ in their spatial arrangement due to the rotation of single bonds.
It is important to note that some sources provide conflicting information, stating that constitutional isomers have different molecular formulas while conformational isomers have the same molecular formula. However, the majority of sources, including those focused on organic chemistry, support the explanation provided above.
Constitutional Freedom of Speech: A Global Overview
You may want to see also

Constitutional isomers have different connectivity
Constitutional isomers, also known as structural isomers, share the same molecular formula but have different connectivity between atoms within the molecule. This means that the atoms are connected in different ways, leading to different structural formulas. For example, cyclohexane and methylcyclopentane are constitutional isomers. They have the same atoms but differ in the bonds between them.
Constitutional isomers can be further divided into positional and functional group isomers. Positional isomers have the same connectivity, except for the position of one functional group, which varies. Functional group isomers, on the other hand, have different functional groups, which usually leads to different chemical or physical properties.
Conformational isomers, on the other hand, have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. These are different forms of the same molecule that can interconvert by rotation around single bonds. For example, butane can have constitutional isomers like isobutane, while its conformational isomers include staggered and eclipsed forms.
In organic chemistry, two molecules that can be superimposed on each other through rotation of bonds (conformational changes) or through rotation of the molecule itself are considered to be the same molecule, or "identical copies of the same molecule".
To summarise, the key difference between constitutional and conformational isomers is that constitutional isomers have different connectivity between atoms, resulting in different structural formulas, while conformational isomers have the same connectivity and molecular formula but differ in the spatial orientation of atoms due to rotation around single bonds.
Factual Judgments: Restating the Obvious?
You may want to see also
Explore related products

Conformational isomers have the same connectivity
Conformational isomers have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. They are different forms of the same molecule that can interconvert by rotation around single bonds. For example, butane's conformational isomers include staggered and eclipsed forms.
Conformational isomers are a type of stereoisomer, which have the same connectivity but are oriented differently in three-dimensional space. Stereoisomers can be further divided into configurational isomers (enantiomers and diastereomers) and conformational isomers. Enantiomers are chiral molecules that are non-superimposable mirror images of each other. They have the same connectivity and physical properties, but they differ in their optical properties. Diastereomers are not mirror images of each other and can have different physical properties and chemical reactivities. They arise when a molecule has two or more chiral centers, leading to various possible spatial arrangements. Geometric isomers, or cis-trans isomers, are a type of diastereomer. They differ from other configurational isomers based on their spatial arrangement around a double bond or ring structure.
Conformational isomers can be distinguished from constitutional isomers by focusing on connectivity. Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of atoms within the molecule. This means the atoms are connected in different ways, leading to different structural formulas. For example, cyclohexane and methylcyclopentane are constitutional isomers.
In organic chemistry, two molecules that can be superimposed on each other, through rotation of bonds (i.e. conformational changes) or through rotation of the molecule itself, are considered to be the same molecule. In other words, molecules that are “identical twins” are not isomers; they are considered to be identical copies of the same molecule.
Ethiopian Constitutions: A Comparison of the 1987 and 1995 Versions
You may want to see also

Constitutional isomers are also known as structural isomers
Isomers are compounds with the same molecular formula but different structures or arrangements.
Constitutional isomers, also known as structural isomers, have the same molecular formula but differ in the connectivity of atoms within the molecule. This means that constitutional isomers have the same atoms but are connected in different ways, leading to different structural formulas. For example, cyclohexane and methylcyclopentane are constitutional isomers.
Constitutional isomers can be further subdivided into positional and functional group isomers. Positional isomers have the same connectivity, except the position of one functional group varies. Functional group isomers, on the other hand, have different functional groups, which usually leads to different chemical or physical properties.
Conformational isomers, on the other hand, have the same molecular formula and connectivity but differ in the spatial orientation of atoms due to rotation around single bonds. These are different forms of the same molecule that can interconvert by rotation around single bonds. For instance, butane can have constitutional isomers like isobutane, while its conformational isomers include staggered and eclipsed forms.
To differentiate between constitutional and conformational isomers, focus on the connectivity of atoms for constitutional isomers and the rotation around single bonds for conformational isomers.
The Constitution's Exclusionary Origins: A Historical Overview
You may want to see also
Frequently asked questions
Constitutional isomers, also known as structural isomers, are compounds with the same molecular formula but different structures or arrangements. This means that constitutional isomers have the same atoms but are connected in different ways, leading to different structural formulas.
Conformational isomers, also known as conforms, are molecules with the same molecular formula and connectivity but different spatial arrangements due to the rotation of single bonds. These isomers tend to have very similar energies and exist in equilibrium with each other.
Constitutional isomers have different connectivities, while conformational isomers have the same connectivity but differ in spatial orientation due to the rotation of single bonds.

































