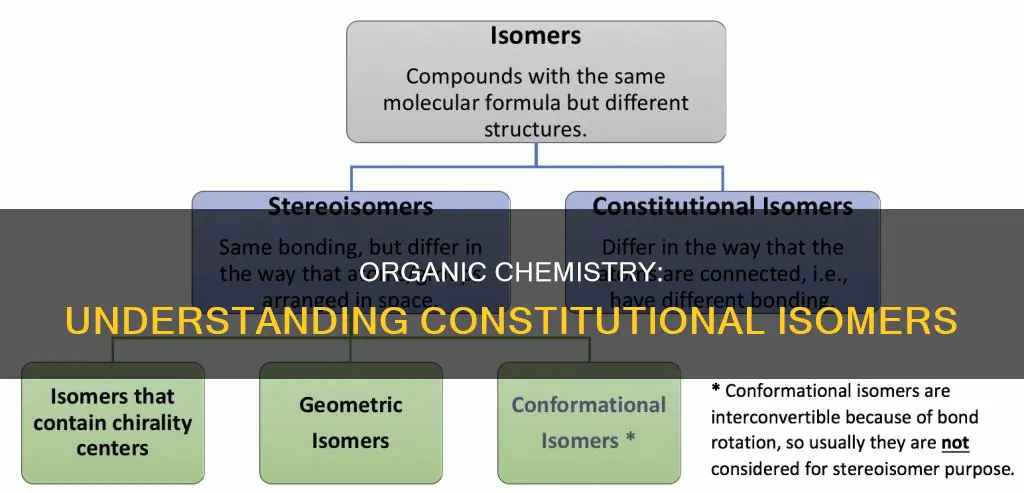
Constitutional isomers, also known as structural isomers, are molecules that have the same molecular formula but differ in their bonding atomic organization and bonding patterns. In other words, they have the same types and numbers of atoms but differ in the way these atoms are connected. Constitutional isomers are commonly found in organic compounds with long carbon chains, such as alkanes. For example, pentane, an alkane with five carbon atoms, can be rearranged in three different ways, resulting in three different constitutional isomers: n-pentane, isopentane, and neopentane. Understanding constitutional isomers is crucial in organic chemistry, as it helps in distinguishing between different molecules with varying properties.
| Characteristics | Values |
|---|---|
| Definition | Constitutional isomers are molecules that have the same molecular formula but differ in their bonding atomic organization, bonding patterns, and connectivity of atoms. |
| Other Names | Structural isomers, functional isomers |
| Types | Skeletal isomers (chain isomers), functional isomers |
| Examples | 1-hexene and cyclohexane, ethanol (ethyl alcohol) and dimethyl ether, pentane and its three isomers (n-pentane, isopentane, and neopentane) |
| HDI Index | Constitutional isomers have the same HDI index as they share the same molecular formula. |
Explore related products
What You'll Learn

Constitutional isomers are molecules with the same molecular formula but different atomic connectivity
In organic chemistry, constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but differ in their atomic connectivity and bonding patterns. These isomers can be understood as different structural arrangements of the same set of atoms, resulting in distinct molecular structures with unique properties.
To illustrate this concept, let's consider the example of ethanol (ethyl alcohol) and dimethyl ether, which are constitutional isomers with the molecular formula C2H6O. While both molecules have the same types and ratios of atoms, the connections between these atoms differ. In other words, the constitution or arrangement of the molecule varies, making them distinct molecular entities with unique characteristics.
Constitutional isomers can be further classified into several types, including skeletal isomers (chain isomers) and functional isomers. Skeletal isomers arise from different orderings of the molecule's skeleton, commonly occurring in organic compounds with long carbon chains. For instance, pentane, an alkane with five carbon atoms, can be rearranged into three different chain isomers: n-pentane, iso-pentane, and neo-pentane. Functional isomers, on the other hand, share the same molecular formula but exhibit variations in how their atoms are connected. A notable example of functional isomerism is seen in 1-hexene and cyclohexane. 1-hexene has a straight-chain structure with a carbon-carbon double bond, while cyclohexane possesses a cyclic structure without any carbon-carbon double bonds.
The identification and understanding of constitutional isomers are essential in organic chemistry. The concept highlights the intricate relationship between molecular structure and properties. By manipulating the connectivity of atoms within a given molecular formula, scientists can explore the diverse chemical behaviours and characteristics exhibited by these isomers.
In summary, constitutional isomers in organic chemistry represent molecules with the same molecular formula but distinct atomic connectivity. They offer valuable insights into the complex nature of chemical bonding and molecular diversity, contributing significantly to our understanding of organic compounds and their behaviour.
The Constitution's Body: Framing Our Nation's Future
You may want to see also

They are also known as structural isomers
In organic chemistry, constitutional isomers, also known as structural isomers, refer to compounds that have the same molecular formula but differ in the way their atoms are connected or arranged structurally. The term "constitutional" refers to the constitution or makeup of the compound, emphasizing that these isomers have different arrangements of atoms and thus, different chemical structures.
The key aspect that distinguishes constitutional isomers from other types of isomers is the difference in the connectivity of atoms. This means that the atoms are bonded in different orders or configurations, leading to unique arrangements of atoms in space. As a result, constitutional isomers have distinct physical and chemical properties, often including differences in boiling points, melting points, solubilities, and chemical reactivities.
The concept of constitutional isomers arises primarily in organic compounds, where the presence of carbon atoms and their ability to form long chains or rings, as well as the presence of various functional groups, allows for numerous possible arrangements of atoms, even when the number and type of atoms remain the same. For example, in the case of butane (C4H10), there are two possible isomers: n-butane (normal butane) and isobutane (methylpropane). The difference in their structures lies in how the carbon atoms are connected: n-butane has a straight-chain structure, while isobutane has a branched-chain structure.
The term "structural isomer" explicitly highlights this difference in atomic connectivity. It emphasizes that the isomers have distinct structural formulas, meaning the atoms are connected in different ways, leading to unique molecular geometries. This term is particularly useful when contrasting constitutional isomers with stereoisomers, which have the same constitutional structure but differ only in the spatial arrangement of atoms or functional groups around a chiral center or double bond.
The existence of constitutional isomers underscores the importance of chemical structure in determining the properties and behaviors of compounds. Even a slight change in the arrangement of atoms can lead to significant differences in a compound's physical state (solid, liquid, or gas), reactivity with other compounds, and biological activity. Understanding constitutional isomers is crucial in fields such as pharmacology, where slight modifications in molecular structure can lead to dramatic changes in a drug's effectiveness or toxicity.
In summary, constitutional isomers, also known as structural isomers, are organic compounds with the same molecular formula but different arrangements of atoms and bonding patterns. The term "structural isomer" emphasizes the unique connectivity of atoms in these compounds, leading to distinct physical and chemical properties. The concept of constitutional isomers highlights the significance of chemical structure in organic chemistry and its impact on the behavior and reactivity of compounds.
The Constitution and Capitalism: A Direct Connection?
You may want to see also

They are distinct from stereoisomers
In organic chemistry, constitutional isomers, also known as structural isomers, are compounds that have the same molecular formula but differ in the way their atoms are connected or arranged in space. This means that constitutional isomers have the same number of each type of atom (e.g., carbon, hydrogen, oxygen), but the atoms are connected in a different order or arrangement. These isomers are an important concept in organic chemistry as they highlight the diversity of organic compounds and the complexity that arises from the ability of carbon atoms to form multiple bonds and chains.
Now, it's important to distinguish constitutional isomers from stereoisomers. While both types of isomers have the same molecular formula, the key difference lies in how their atoms are arranged in space. Constitutional isomers have different connectivities of atoms, meaning the atoms are bonded in a different order, forming distinct chemical structures. On the other hand, stereoisomers have the same connectivity of atoms but differ in the way these atoms are oriented in three-dimensional space.
Stereoisomers can be further categorized into two main types: cis-trans isomers (or geometric isomers) and enantiomers (or optical isomers). Cis-trans isomers occur when there is a restricted rotation around a double bond, leading to different spatial arrangements of atoms or functional groups attached to the double bond. Enantiomers, on the other hand, are stereoisomers that are mirror images of each other and are non-superimposable, much like a person's right and left hands. Enantiomers often have different biological activities, with one form being biologically active while the other may be inactive or even harmful.
The distinction between constitutional and stereoisomers is crucial because they exhibit different chemical and physical properties. Constitutional isomers, due to their different atomic connectivities, can have vastly different chemical reactivities, solubilities, boiling points, and melting points. Stereoisomers, on the other hand, often have very similar chemical properties, but their physical properties can differ significantly, especially in the case of enantiomers. This is why stereoisomers play a critical role in fields like pharmacology, where the effectiveness and safety of a drug can depend on its stereochemical configuration.
To summarize, constitutional isomers are distinct from stereoisomers in that they have different atomic connectivities, resulting in unique chemical structures. Stereoisomers, while having the same connectivity, differ only in the spatial arrangement of atoms in three-dimensional space. Understanding this distinction is essential in organic chemistry, as it forms the basis for predicting and understanding the diverse properties and behaviors of organic compounds. This knowledge also has practical applications in various fields, including pharmaceuticals, where the stereochemical configuration of a molecule can have profound implications for its biological activity and safety.
The Long Road to Ratifying the Constitution
You may want to see also
Explore related products

Examples include ethanol and dimethyl ether
Constitutional isomers are compounds that share the same molecular formula but differ in their structural formulas or bonding arrangements. They are named so because of the Greek origin of the word "isomer", which means "same things", referring to their identical content. However, they often have very different physical and biological properties.
Ethanol and dimethyl ether are constitutional isomers with the formula C2H6O. They have different physical properties. At room temperature, dimethyl ether is a gas, while ethanol is a liquid. This is because the boiling point of dimethyl ether is lower than room temperature, while ethanol's freezing point is much lower than room temperature and its boiling point is higher.
The difference in their boiling points can be attributed to the difference in intermolecular attractions. In ethanol, an alcohol, there is an OH group attached to a carbon. This allows for hydrogen bonding, which is a strong intermolecular force. On the other hand, dimethyl ether, an ether, has an O attached to two carbons, resulting in regular dipole moments as the major intermolecular attraction. Since hydrogen bonds are typically stronger than ordinary dipole moments, a group of ethanol molecules is harder to separate than a group of dimethyl ether molecules, resulting in ethanol's higher boiling point.
Another example of constitutional isomers with the formula C3H9N includes three isomeric amines that differ in their melting and boiling points due to the presence or absence of hydrogen bonding.
The Constitution and Cabinet Formation
You may want to see also

They can be identified using the HDI index
Constitutional isomers, also called structural isomers, are molecules with the same molecular formula but different bonding arrangements or connectivity between atoms. For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers. They have the same atoms in the same ratios, but the connections between those atoms are different, resulting in different properties.
The HDI index, or the Index of Hydrogen Deficiency, is a tool that can be used to identify constitutional isomers. By calculating the HDI, we can gain insights into the structural motifs that a molecule may possess. A higher HDI indicates a greater number of possibilities for structural motifs, while a lower HDI suggests a more limited range of structural options.
To illustrate this, let's consider a molecule with the molecular formula C3H6O. By determining the HDI, we can deduce that this molecule must contain either a double bond or a cycle, but not both, since the HDI equals 1. This information helps us narrow down the potential structural configurations and guide our exploration of possible constitutional isomers.
While the HDI is a valuable tool, it does not provide an "instant answer," especially for more intricate molecules. It serves as a starting point, and further analysis is required to fully understand the molecule's structure. The complexity increases when considering stereochemistry, which introduces additional factors that influence the molecule's properties.
In summary, the HDI index is a potent tool for identifying constitutional isomers in organic chemistry. It provides insights into the structural possibilities of molecules, guiding the process of exploring and understanding their isomeric forms. However, it is just one aspect of the broader challenge of distinguishing between molecules with similar structures yet vastly different properties.
Missouri's Constitution: Effective Date and Beyond
You may want to see also
Frequently asked questions
Constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but have different bonding atomic organisation and bonding patterns.
The two molecules ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers. They have the same atoms in the same ratios in the molecule but differ in the connections between those atoms.
The three prominent types of constitutional isomers are skeletal isomers (or chain isomers), functional isomers, and positional isomers.
Constitutional isomers have different connectivities, whereas stereoisomers have the same connectivity but differ in the arrangement of their atoms in space.








![Organic Chemistry: Official OpenStax by John McMurry 10th Ed [hardcover, full color]](https://m.media-amazon.com/images/I/51X6FFr6TML._AC_UL320_.jpg)


































