In organic chemistry, isomers are two or more molecules that share the same molecular formula but differ in their atomic organization and bonding patterns. These are known as constitutional isomers, also referred to as structural isomers. They are distinct from stereoisomers, which have the same connectivity but differ in the arrangement of their atoms in space. Constitutional isomers can be further categorized into skeletal isomers (chain isomers) and functional isomers, which differ in how atoms are connected to each other. For example, 1-hexene and cyclohexane have the same molecular formula, but 1-hexene has a straight-chain structure with a carbon-carbon double bond, while cyclohexane has a cyclic structure without any carbon-carbon double bonds. Understanding constitutional isomers is crucial in organic chemistry, as it helps identify different molecules and their unique properties.
| Characteristics | Values |
|---|---|
| Definition | Two molecules which have the same molecular formula but different structural formulas, or bonding arrangements |
| Other names | Structural isomers |
| Types | Skeletal isomers (chain isomers), functional isomers |
| Examples | 1-hexene and cyclohexane, ethanol (ethyl alcohol) and dimethyl ether, pentane and its three isomers |
| Approach | Start with the possible linear structures, then add the necessary number of hydrogens, being careful not to add too many and maintaining bonding patterns |
| HDI | The combination of cycles and double or triple bonds in a molecule will give you the HDI value, which is useful for constitutional isomers as they have the same molecular formula and HDI indexes |
Explore related products
What You'll Learn
- Constitutional isomers are molecules with the same molecular formula but different atomic connectivity
- Examples include ethanol and dimethyl ether, which are constitutional isomers of C2H6O
- Constitutional isomers are also known as structural isomers
- They can be categorised as skeletal isomers, commonly known as chain isomers
- Functional isomers are constitutional isomers that vary in how atoms are connected

Constitutional isomers are molecules with the same molecular formula but different atomic connectivity
In organic chemistry, constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but differ in their atomic connectivity and bonding patterns. This means that the atoms within the molecules are connected differently, resulting in distinct molecular structures.
For example, let's consider the molecule with the molecular formula C2H6O. This formula represents two possible connections between atoms: ethanol (ethyl alcohol) and dimethyl ether. These two molecules are examples of constitutional isomers. They contain the same number of atoms in identical ratios, but the connections between those atoms differ, resulting in distinct molecular constitutions.
Another example is the comparison between 1-hexene and cyclohexane. Both isomers share the same molecular formula but exhibit variations in atomic connectivity. Specifically, 1-hexene possesses a straight-chain structure with a single carbon-carbon double bond, while cyclohexane displays a cyclic structure devoid of any carbon-carbon double bonds.
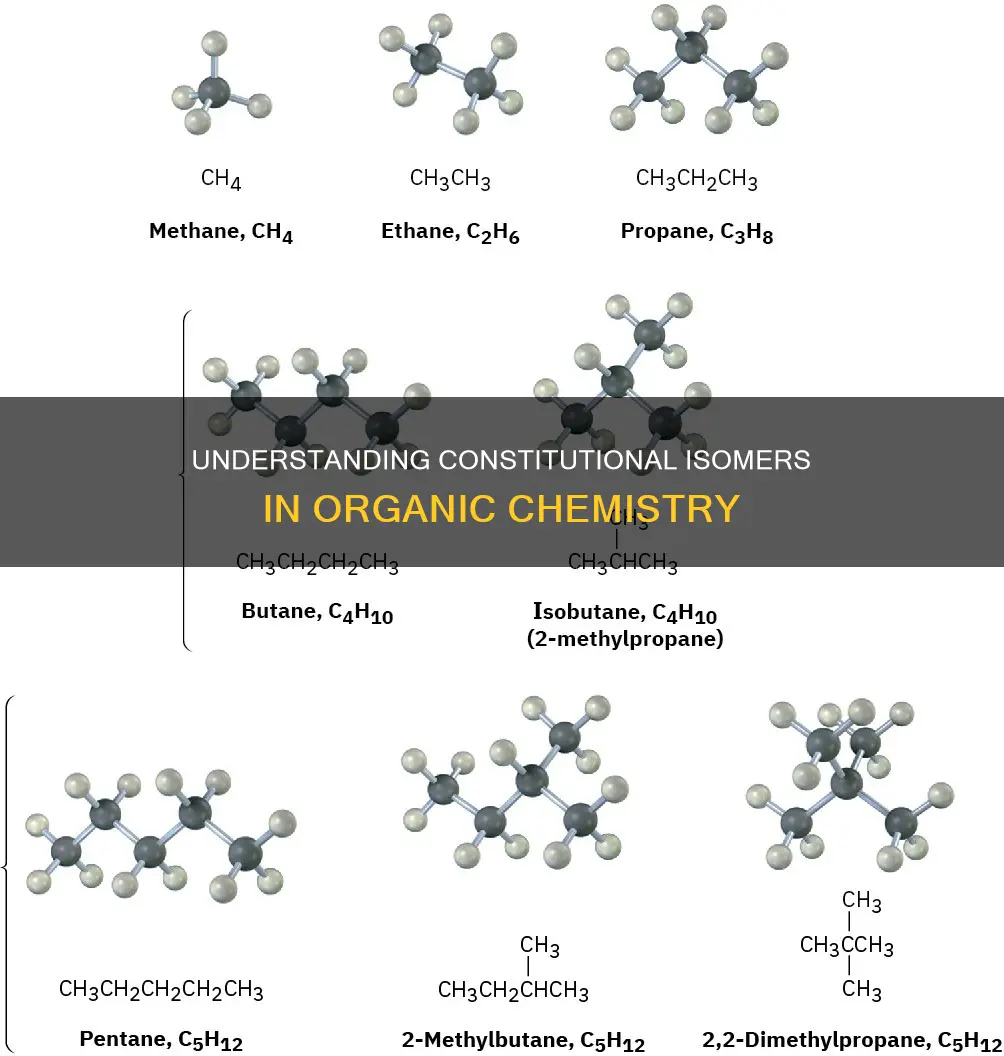
The concept of constitutional isomers is particularly relevant in organic compounds containing long carbon chains, such as alkanes. For instance, pentane, an alkane with five carbon atoms, can be rearranged in three different ways, yielding three distinct chain isomers: n-pentane, iso-pentane, and neo-pentane. These isomers possess the same molecular formula but differ in the ordering of atoms within their structures.
Constitutional isomers are distinguished from stereoisomers, which share the same atomic connectivity but exhibit different arrangements of their atoms in space. It is important to recognize that two molecules can be stereoisomers of each other but cannot simultaneously be stereoisomers and constitutional isomers. This distinction is crucial in understanding the structural diversity of molecules in organic chemistry.
Managing Factions: The Constitution's Guide to Unity
You may want to see also

Examples include ethanol and dimethyl ether, which are constitutional isomers of C2H6O
Constitutional isomers are two molecules that share the same molecular formula but differ in their structural formulas or bonding arrangements. They are also known as structural isomers.
Ethanol (C2H5OH) and dimethyl ether (CH3OCH3) are constitutional isomers of C2H6O. They have the same chemical formula but differ in their structures and physical properties. At room temperature, dimethyl ether is a gas, whereas ethanol is a liquid. This difference arises due to the variation in their intermolecular attractions. Dimethyl ether molecules interact through regular dipole moments, while ethanol molecules exhibit hydrogen bonding, which is a stronger force. Consequently, ethanol has a much higher boiling point than dimethyl ether.
The difference in intermolecular forces between these isomers extends to their respective groups of molecules. A group of ethanol molecules is more challenging to separate compared to a group of dimethyl ether molecules due to the stronger hydrogen bonds between ethanol molecules.
Another example of constitutional isomers with the formula C3H9N includes compounds with different melting and boiling points. The presence or absence of hydrogen bonding capabilities in these isomers influences their physical properties.
Roger Sherman's Influence on the US Constitution
You may want to see also

Constitutional isomers are also known as structural isomers
Constitutional isomers, also known as structural isomers, are specific types of isomers that share the same molecular formula but have different bonding atomic organisation and bonding patterns. In other words, they have the same types and numbers of atoms but differ in the way these atoms are connected. This is also known as connectivity.
For example, ethanol (ethyl alcohol) and dimethyl ether are constitutional isomers with the molecular formula C2H6O. They have the same atoms in the same ratios, but the connections between those atoms differ. This results in two different molecules with distinct properties.
Constitutional isomers can be further categorised into skeletal isomers (also known as chain isomers) and functional isomers. Skeletal isomers are constitutional isomers in which the components of the molecule's skeleton are ordered differently, creating distinct skeletal structures. This type of isomerism is common in organic compounds with long carbon chains, such as pentane, which can be rearranged into three different chain isomers: n-pentane, isopentane, and neopentane.
On the other hand, functional isomers share the same molecular formula but vary in how the atoms are connected to each other. An example of functional isomerism is 1-hexene and cyclohexane. 1-hexene has a straight-chain structure with one carbon-carbon double bond, while cyclohexane has a cyclic structure with no carbon-carbon double bonds.
It is important to note that while constitutional isomers have different connectivities, stereoisomers are isomers that have the same connectivity but differ in the arrangement of their atoms in space. Constitutional isomers and stereoisomers are distinct categories, and a pair of molecules cannot be both constitutional and stereoisomers.
The Ever-Expanding Constitution: World's Longest
You may want to see also
Explore related products

They can be categorised as skeletal isomers, commonly known as chain isomers
Isomerism is a phenomenon in which multiple compounds share the same chemical formula but differ in chemical structure and properties. These compounds are called isomers. The word "isomer" comes from the Greek words "isos" and "meros", meaning "equal parts". Isomers can be categorised as skeletal isomers, commonly known as chain isomers.
Chain isomers have the same molecular formula but differ in the way their carbon atoms are joined. Alkane molecules with four or more carbon atoms can have chain isomers. These isomers are bonded together in different ways, resulting in branching. For example, pentane (C5H12) has three chain isomers. One is an unbranched "straight chain" isomer, while the other two are branched. Each branch contains a single carbon atom, forming a methyl group (-CH3).
The structural isomerism observed in chain isomers arises from the possibility of branching in carbon chains. For instance, butane (C4H10) has two isomers. One isomer has carbon atoms in a straight chain, while the other has a branched structure. It is important to distinguish these true isomers from "false" isomers, which are simply twisted versions of the original molecule.
Another example of chain isomerism is observed in compounds with the formula C3H7Cl. This type of isomerism is called functional group isomerism, where the functional groups are attached to different carbon atoms in the carbon chain. A similar phenomenon is observed in ring-chain isomerism, where one isomer has an open-chain structure, while the other has a ring structure. For example, C3H6 has two isomers: propene (open-chain) and cyclopropane (ring structure).
Who's the Most Powerful Person in Congress?
You may want to see also

Functional isomers are constitutional isomers that vary in how atoms are connected
Constitutional isomers are molecules with the same molecular formula but different bonding arrangements or structures. They are also known as structural isomers. In organic chemistry, functional isomers are a type of constitutional isomer.
Functional isomers have the same molecular formula but different functional groups. These functional groups are groupings of atoms that are connected in different ways. For example, butanol and methylpropanol both have the molecular formula C4H10O, but differ in their functional groups. Butanol has a continuous chain of carbon atoms, while in methylpropanol, a methyl group is substituted on the second carbon atom.
The different functional groups in functional isomers can dramatically alter the physical and chemical properties of the molecule, including its reactivity, stability, polarity, and intermolecular forces. This means that functional isomers can have entirely different characteristics and reactivity, despite having the same molecular formula.
Functional group isomerism is a rare type of isomerism, generally limited to molecules that contain a divalent atom, such as sulphur or oxygen, surrounded by alkyl groups. An example of functional group isomerism is the compound C3H6O, which can be represented as ethoxyethane (C2H5OC2H5) and methoxy-propane (CH3OC3H7).
In summary, functional isomers are constitutional isomers that vary in how atoms are connected to form different functional groups. These functional groups can significantly impact the properties and reactivity of the molecule.
Religious Freedom: Constitutional Limits on Religion in America
You may want to see also
Frequently asked questions
Constitutional isomers, also known as structural isomers, are molecules that share the same molecular formula but have different bonding atomic organisation and bonding patterns.
The three prominent types of constitutional isomers are skeletal isomers (or chain isomers), functional isomers, and positional isomers.
To identify constitutional isomers, it is important to first determine the possible linear structures. Then, add the necessary number of hydrogens while being careful not to add too many and maintaining consistency with bonding patterns.











































