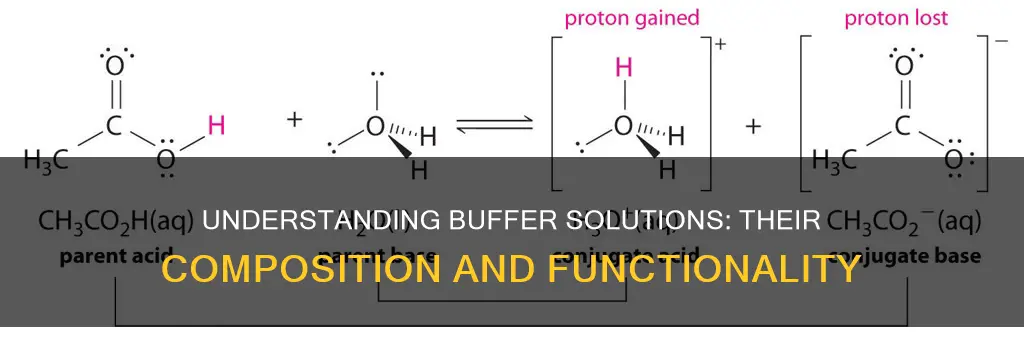
A buffer solution is a solution that can resist changes in pH levels upon the addition of an acid or base. It is able to neutralize small amounts of added acid or base, thus maintaining a relatively stable pH. This is important for processes that require a specific and stable pH range. Buffer solutions have a working pH range and capacity, which dictate how much acid or base can be neutralized before the pH changes. To effectively maintain a pH range, a buffer must consist of a weak conjugate acid-base pair, meaning either a weak acid and its conjugate base or a weak base and its conjugate acid.
Explore related products
What You'll Learn

Buffer solutions are a mixture of a weak acid and its conjugate base
A buffer solution is a solution that can resist changes in pH levels upon the addition of a small amount of acid or alkali. It does so by neutralizing the added acid or base, thereby maintaining a moderate pH.
Buffers function best when the pKa of the conjugate weak acid used is close to the desired working range of the buffer. This is achieved when the concentrations of the conjugate acid and conjugate base are approximately equal (within about a factor of 10). The Henderson-Hasselbalch equation can be used to calculate the pH of a buffer solution prepared by controlling the salt-acid or the salt-base ratio. The equation is popularly known as the Henderson equation and is used to calculate the ratio of the base to acid when pH = pKa in a buffer solution.
The buffer capacity of a solution is the amount of acid or base that can be added before the pH begins to change significantly. It is defined as the quantity of strong acid or base that must be added to change the pH of one liter of the solution by one pH unit. The capacity of a buffer solution is reached when the entire base and its conjugate acid are consumed to neutralize the added acid or base. Once the buffering capacity is exceeded, the rate of pH change increases dramatically.
Public Access: Exploring Constitutional Boundaries
You may want to see also

Or, a weak base and its conjugate acid
A buffer solution is a water-based solvent solution that can resist changes in pH levels when a small amount of acid or base is added. In other words, it is a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. The pH of the buffer solution remains relatively stable even when a small amount of strong acid or base is added.
Buffers work by neutralizing any added acid (H+ ions) or base (OH- ions) to maintain a moderate pH, making them a weaker acid or base. For example, when a strong acid like HCl is added to a buffer system of a weak base like ammonia (NH3) and its conjugate acid (NH4+), the extra H+ ions are consumed by the NH3 to form NH4+. This results in a stable pH.
The Henderson-Hasselbalch equation, also known as the Henderson equation, can be used to calculate the pH of a buffer solution prepared from a mixture of a weak base and its conjugate acid. The equation is as follows:
PH = pKa + log ( [conjugate acid]/ [weak base])
Where:
- PH is the desired pH of the buffer solution
- PKa is the negative logarithm of the acid dissociation constant (Ka) of the weak base
- [conjugate acid] is the concentration of the conjugate acid in the buffer solution
- [weak base] is the concentration of the weak base in the buffer solution
By manipulating the concentrations of the weak base and its conjugate acid, you can prepare a buffer solution with the desired pH. The Henderson-Hasselbalch equation is not applicable to strong acids and bases.
It is important to note that the buffer solution has a limited capacity to neutralize added acids or bases. This capacity is known as the "breaking of the buffer solution." Once the capacity is reached, further addition of an acid or base will result in a rapid change in pH. Therefore, it is crucial to consider the amount of acid or base added to maintain the stability of the buffer solution.
Whiskey Rebellion: Constitutional or Not?
You may want to see also

They resist changes in pH upon dilution
Buffer solutions are aqueous solutions that can resist changes in pH levels upon dilution or the addition of small amounts of acid or alkali. They are made up of a weak acid and its conjugate base or a weak base and its conjugate acid. The weak acid is partly ionised, while the salt is completely ionised.
Buffers work by neutralizing any added acid (H+ ions) or base (OH- ions) to maintain a moderate pH, making them a weaker acid or base. For example, when HCl (a strong acid) is added to a buffer system of weak base ammonia (NH3) and its conjugate acid (NH4+), the extra H+ ions are consumed by the NH3 to form NH4+. This results in a relatively stable pH.
Buffers have a working pH range and capacity, which determine how much acid or base can be neutralized before the pH changes. The Henderson-Hasselbalch equation can be used to calculate the pH of a buffer solution. The buffer capacity is the amount of acid or base that can be added before the pH begins to change significantly.
Buffers are essential in biological systems as they help maintain a relatively constant pH. For instance, the CO2/HCO3- buffer system is crucial for the buffering action of blood plasma.
The Constitution's Citizen Mentions: A Comprehensive Count
You may want to see also
Explore related products
$19.99

Or, when a small amount of acid/alkali is added
A buffer solution is a solution that can resist changes in pH when an acid or base is added to it. This is due to the presence of a weak acid and its conjugate base (or a weak base and its conjugate acid) in the solution, which act as a pH buffer.
When a small amount of acid or alkali is added to a buffer solution, the pH of the solution changes slightly, but not significantly. The weak acid or base in the buffer reacts with the added acid or alkali, preventing a large change in pH. For example, if a small amount of hydrochloric acid (HCl) is added to a buffer solution of acetic acid (CH3COOH) and its conjugate base acetate (CH3COO-), the HCl reacts with the CH3COO- ions to form CH3COOH. This reaction consumes the HCl, preventing a large decrease in pH. Similarly, if a small amount of sodium hydroxide (NaOH) is added, it reacts with CH3COOH to form CH3COO- and water, consuming the NaOH and preventing a large increase in pH.
The key to the effectiveness of buffer solutions is the presence of the weak acid and its conjugate base (or weak base and its conjugate acid) in specific concentrations. The pH of the solution is determined by the pKa of the weak acid and the ratio of the concentrations of the weak acid to its conjugate base. By adjusting these concentrations, buffer solutions can be designed to maintain a specific pH.
The buffer capacity, or the ability of a buffer solution to resist changes in pH, depends on the concentrations of the buffering components. A buffer solution with a high concentration of the weak acid and its conjugate base will have a higher buffer capacity than one with low concentrations. Additionally, the pH range over which a buffer is effective is centered on the pKa of the weak acid or base. It is most effective at maintaining pH in this range.
Buffer solutions are essential in various chemical and biological applications. They are used in laboratories to maintain stable pH conditions during experiments, in
Majority Rule Limits: The Constitution's Checks and Balances
You may want to see also

Buffer solutions have a working pH range and capacity
Buffer solutions are designed to maintain a stable pH level, even when small amounts of acid or alkali are added. However, they have a limited capacity, and once this capacity is exceeded, the pH can change rapidly. This capacity is determined by the buffer's composition and pH range.
The capacity of a buffer solution is defined as the amount of strong acid or base that can be added to one litre of the solution before the pH changes by one unit. In other words, it is the amount of acid or base that the buffer can absorb before its capacity is exceeded. This capacity is influenced by the concentrations of the conjugate acid and base in the solution. When the conjugate acid or base is depleted through neutralisation, the buffer's capacity is reached, and further addition of an acid or base will result in a rapid pH change.
The working pH range of a buffer solution refers to the range of pH values within which the solution can effectively neutralise added acids or bases while maintaining a relatively constant pH. This range is determined by the specific buffer components and their concentrations. Different buffer solutions have different pH ranges, and the pH of a buffer solution can be adjusted by changing the ratio of the acid to the base or by choosing different acids and bases.
The Henderson-Hasselbalch equation is used to calculate the pH of a buffer solution prepared from a mixture of a weak acid and its conjugate base or a weak base and its conjugate acid. The equation takes into account the acidity constant of the weak acid, the concentrations of the acidic and basic forms, and the activity coefficients of the species involved. By manipulating this equation, the pH of the buffer solution can be adjusted to the desired range.
The buffer capacity and pH range are crucial in various applications, such as in biological systems, where enzymes require a specific pH range to function correctly. For example, the bicarbonate buffering system in human blood helps maintain a pH between 7.35 and 7.45. Outside this range, metabolic conditions like acidosis and alkalosis can develop, leading to severe health issues.
Inground Pool Installation: What You Need to Know
You may want to see also
Frequently asked questions
A buffer solution is a solution that can resist a significant change in pH levels upon the addition of a small amount of acid or alkali. It is a water-based solvent that consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
The pH of a buffer solution is typically maintained within a narrow range, which can vary depending on the specific buffer solution. Acidic buffer solutions have a pH of less than 7, while basic buffer solutions have a pH greater than 7.
Some common examples of buffer solutions include a mixture of acetic acid and sodium acetate, ammonia and ammonium chloride, and phosphate buffered saline (PBS), which is often used in biological research. Other examples include McIlvaine's buffer solutions, which have a pH range of 3 to 8, and the Carmody buffer and Britton-Robinson buffer, developed in 1931. Additionally, there are specialized buffer solutions such as metal buffers and redox buffers, which are used for specific purposes.