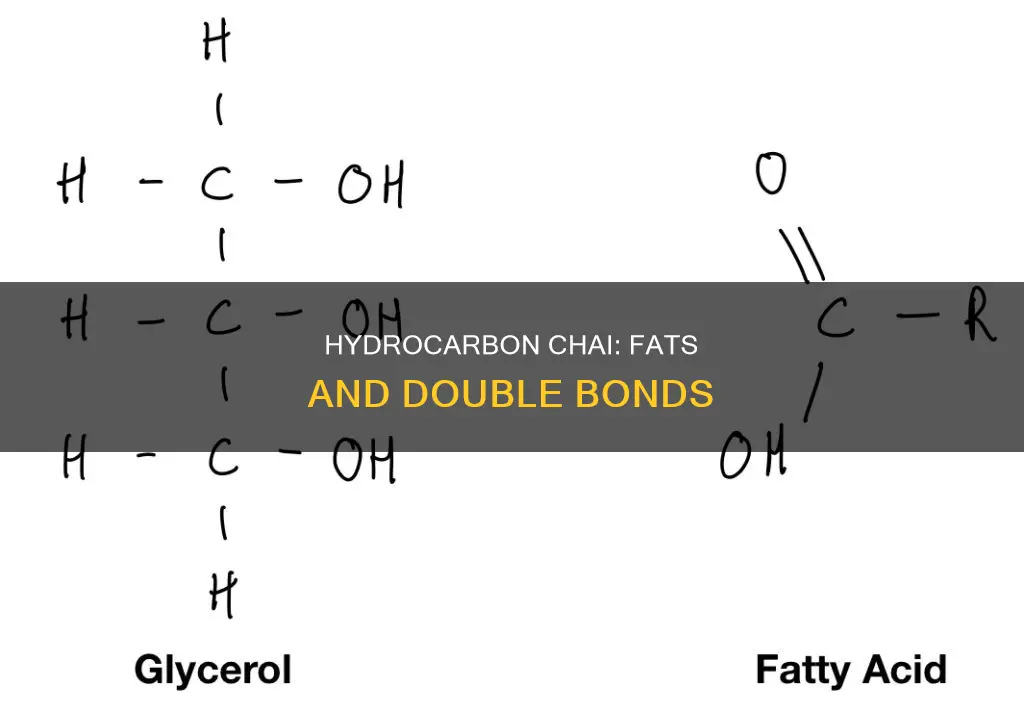
Fatty acids are long chains of carbon atoms bonded together, with hydrogen atoms attached to the carbons. They are a major component of lipids and are either saturated or unsaturated. Saturated fatty acids are saturated with hydrogen and have only single bonds in the hydrocarbon chain. When the hydrocarbon chain contains a double bond, the fatty acid is said to be unsaturated. Unsaturated fatty acids have one or more double bonds, which can be in a cis or trans configuration. The presence of a cis double bond, where the hydrogen atoms are on the same side of the chain, causes a bend or a kink in the chain, affecting the physical properties of the fat. Linoleic acid, with two double bonds, has a pronounced bend and is an example of an unsaturated fatty acid.
| Characteristics | Values |
|---|---|
| Type of fatty acid | Unsaturated |
| Number of double bonds | One or more |
| Cis configuration | Hydrogen atoms on the same side of the hydrocarbon chain |
| Trans configuration | Hydrogen atoms on opposite sides of the hydrocarbon chain |
| Effect of double bonds | Creates a bend or "kink" in the chain, preventing fatty acids from packing tightly and keeping them liquid at room temperature |
| Examples | Oleic acid, linoleic acid, margarine, some types of peanut butter, shortening |
Explore related products
What You'll Learn

Cis and trans isomers
Fatty acids are long chains of carbon atoms bonded together, with hydrogen atoms attached to the carbons. They can be either saturated or unsaturated. Saturated fatty acids have straight chains with single bonds between carbon atoms, while unsaturated fatty acids have one or more double bonds within the carbon chain. The presence of double bonds in a hydrocarbon chain of a fatty acid introduces kinks or bends in the molecule, affecting its physical properties. These double bonds in unsaturated fatty acids can be in either a cis or trans configuration.
On the other hand, in the trans configuration, the hydrogen atoms are on opposite sides of the hydrocarbon chain. Trans fats are created through a hydrogenation process where gas is bubbled through oils to solidify them, converting the cis-conformation double bonds to trans-conformation double bonds. Examples of artificially hydrogenated trans fats include margarine, certain types of peanut butter, and shortening.
The presence of cis or trans double bonds significantly impacts the properties and health effects of fatty acids. Cis double bonds, with their bends, limit the ability of fatty acids to pack closely, influencing the melting temperature of the membrane or fat. This structural difference also affects the fluidity of the fatty acids, making unsaturated fats with double bonds more flexible and fluid compared to saturated fats.
In summary, cis and trans isomers refer to the arrangement of hydrogen atoms around double bonds in unsaturated fatty acids. Cis isomers have hydrogen atoms on the same side, creating bends in the chain, while trans isomers have hydrogen atoms on opposite sides, resulting in straighter chains. These structural differences influence the properties and health impacts of fatty acids.
US Constitution: Export Taxation Rules and Regulations
You may want to see also

Oleic, linoleic, and α-linolenic acids
Fatty acids are carboxylic acids with an aliphatic chain, which can be either saturated or unsaturated. Saturated fatty acids are saturated with hydrogen and have single bonds that increase the number of hydrogens on each carbon. Stearic acid and palmitic acid, commonly found in meat, are examples of saturated fats. On the other hand, unsaturated fatty acids have one or more double bonds, and each double bond can have either a cis or trans configuration. When a chain has multiple cis bonds, it becomes curved in its most accessible conformations.
Oleic acid, a monounsaturated fatty acid with one double bond, has a "kink" in its structure. Linoleic acid, with two double bonds, has a more pronounced bend, and α-Linolenic acid, with three, favours a hooked shape. These cis bonds limit the ability of fatty acids to pack closely, affecting the melting temperature of the membrane or fat. This is why cis fats are liquid at room temperature.
Linoleic acid is associated with lowering the risk of cardiovascular disease, diabetes, and premature death. Higher levels of linoleic acid are correlated with a lower risk of major cardiovascular events. Additionally, it is present in cockroaches, ants, and bees, and is released upon their death to discourage others from entering the area.
The metabolism of linoleic acid (LA) to arachidonic acid (AA) involves conversion into gamma-linolenic acid (GLA) and subsequent oxidation by various enzymes, resulting in products implicated in human physiology and pathology.
The Constitution's Introduction: A Foundation for Freedom
You may want to see also

Saturated fatty acids
Fatty acids are long-chain hydrocarbons that can be divided into four categories: saturated, monounsaturated, polyunsaturated, and trans fats. Saturated fatty acids are saturated with hydrogen, meaning that they have the maximum number of hydrogen atoms attached to every carbon atom. They have straight shapes due to the presence of only single bonds between carbon atoms, which increases the number of hydrogens on each carbon.
In contrast, unsaturated fatty acids contain one or more C=C double bonds, which can take on a cis or trans configuration. Cis configuration occurs when the two hydrogen atoms adjacent to the double bond stick out on the same side of the chain, causing a "'kink" in the shape. This restricts the conformational freedom of the fatty acid. On the other hand, trans configuration happens when the hydrogen atoms are on opposite sides of the double bond.
Examples of saturated fatty acids include lauric acid, myristic acid, palmitic acid, and stearic acid. Lauric and myristic acids are commonly found in tropical oils like palm kernel and coconut, as well as dairy products. Palmitic and stearic acids are present in meat, eggs, cacao, and nuts.
The consumption of saturated fatty acids has been linked to an increased risk of coronary heart disease and cardiovascular disease. Studies have shown that they can increase low-density lipoprotein (LDL) cholesterol levels, which is a risk factor for heart disease and stroke. However, some studies have also found that certain saturated fatty acids, such as stearic acid, do not appear to raise serum cholesterol levels. Additionally, a 2024 meta-analysis suggested that odd-chain and longer-chain saturated fatty acids were negatively associated with the risk of cardiovascular disease, including stroke.
The World Health Organization, American Heart Association, Health Canada, and other health organizations have recommended reducing or replacing dietary intake of saturated fats with unsaturated fats to lower the risk of cardiovascular diseases.
The Constitution's Shield: Judges and Public Opinion
You may want to see also
Explore related products

Hydrogenation process
Fatty acids are carboxylic acids with an aliphatic chain that can be either saturated or unsaturated. Saturated fatty acids, such as stearic and palmitic acids, commonly found in meat, are saturated with hydrogen due to the presence of single bonds that increase the number of hydrogen atoms on each carbon atom. On the other hand, unsaturated fatty acids have one or more double bonds, with each double bond having a cis or trans configuration. Cis configuration occurs when the hydrogen atoms adjacent to the double bond are on the same side of the chain, causing a bend or a "kink" in the structure. Trans configuration, on the other hand, has hydrogen atoms on opposite sides of the chain.
Hydrogenation is a chemical process that is commonly used to convert unsaturated compounds into saturated derivatives. It involves the addition of hydrogen to the unsaturated bonds on the fatty acid chains, leading to the conversion of liquid oils into semi-solid or solid fats. This process is widely used in the food industry to modify the composition, structure, and consistency of fats and oils, increasing their melting point and oxidative stability.
The hydrogenation process typically involves bubbling gas through oils to solidify them. During this process, the double bonds in the cis-conformation of the hydrocarbon chain may be converted to double bonds in the trans-conformation. This results in the production of trans fats, which are commonly found in margarine, some types of peanut butter, and shortening.
The food industry has been working on reducing the trans fat content in their products due to the negative health effects associated with their consumption. This has led to the development of alternative methods, such as the low-temperature electrocatalytic process and the use of different catalysts, to reduce the formation of trans fats during hydrogenation.
In addition to its applications in the food industry, hydrogenation is also used in petrochemical processes to convert alkenes and aromatics into saturated hydrocarbons, which have superior storage properties and are less toxic and reactive.
The Constitution's Executive Solution: Fixing a Branchless Government
You may want to see also

Triglycerides and phospholipids
Fatty acids are long chains of carbon atoms bonded together, with hydrogen atoms attached to the carbons. They are a major component of fats and can be either saturated or unsaturated. Saturated fatty acids have straight chains with single bonds between carbon atoms and are saturated with hydrogen atoms. When a hydrocarbon chain contains a double bond, the fatty acid is considered unsaturated. These double bonds create gaps where hydrogen atoms are missing, influencing the properties and health effects of the fatty acid. Unsaturated fatty acids are more flexible and fluid due to the bends in the chains, resulting in looser packing compared to saturated fats.
The two types of unsaturated fatty acids are monounsaturated, with one double bond, and polyunsaturated, with two or more double bonds. Oleic acid, with one double bond, has a "kink" in its structure, while linoleic acid, with two double bonds, has a more pronounced bend. α-Linolenic acid, with three double bonds, favours a hooked shape. These double bonds can be in either a cis or trans configuration. Cis configuration occurs when both hydrogen atoms adjacent to the double bond are on the same side of the chain, causing the chain to bend and restricting its conformational freedom. Trans configuration, on the other hand, has hydrogen atoms on opposite sides of the chain.
The presence of double bonds in fatty acids within triglycerides and phospholipids has significant implications. For instance, cis bonds limit the ability of fatty acids to pack closely together, affecting the melting temperature of the membrane or fat. This property is essential in biological systems, influencing the fluidity and functionality of cell membranes. Additionally, the presence of double bonds can impact the health effects of fatty acids. For example, unsaturated fatty acids are associated with positive health outcomes, such as improved blood cholesterol levels and a reduced risk of cardiovascular disease.
Missouri Constitution: Unique Features vs US Constitution
You may want to see also
Frequently asked questions
Fatty acids are long chains of carbon atoms bonded together, with hydrogen atoms attached to the carbons.
Saturated fatty acids are saturated with hydrogen since single bonds increase the number of hydrogens on each carbon. When every carbon atom in the fatty acid chain is bound to as many hydrogen atoms as possible, the fatty acid is termed 'saturated'. Stearic acid and palmitic acid, which are commonly found in meat, are examples of saturated fats.
Unsaturated fatty acids have one or more double bonds. When the hydrocarbon chain contains a double bond, the fatty acid is said to be unsaturated. Oleic acid is an example of a monounsaturated fatty acid.
A fatty acid with single bonds between carbon atoms is called saturated, whereas one with one or more double bonds is called unsaturated. The presence of double bonds in a hydrocarbon chain of a fatty acid introduces kinks or bends in the molecule.
The C=C double bonds in unsaturated fatty acids can give either cis or trans isomers. In the cis configuration, both hydrogens are on the same side of the hydrocarbon chain, whereas in the trans configuration, the hydrogens are on opposite sides.











































