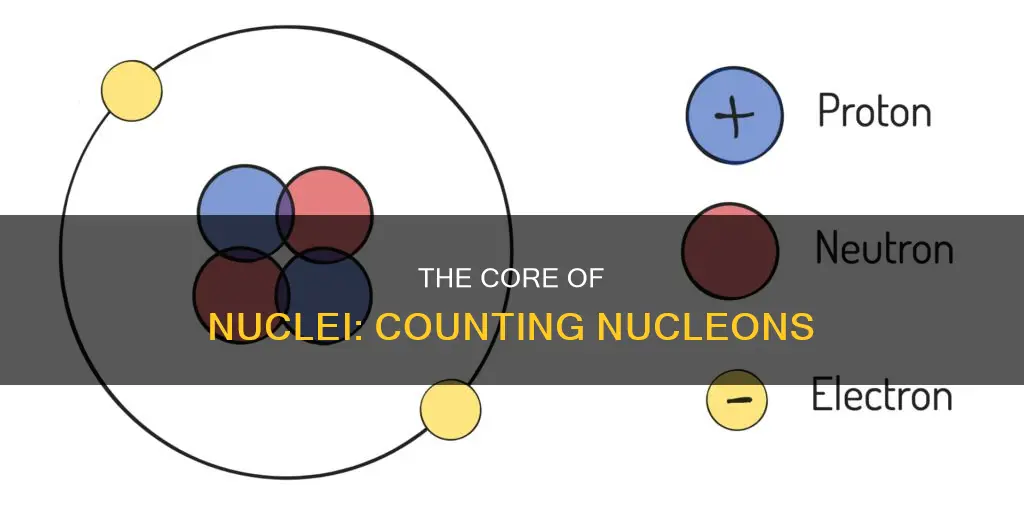
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number. The mass number of a nucleus is equal to the number of neutrons in the nucleus, the number of protons in the nucleus, and the number of nucleons in the nucleus. The atomic number is the number of electrons or protons present in an atom of an element. Mass number is the total number of protons and neutrons present in an atom of an element.
| Characteristics | Values |
|---|---|
| Definition of a nucleon | A nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. |
| Nucleon composition | Nucleons are made of three quarks bound together by the strong interaction. |
| Mass number | The mass number of a nucleus is equal to the number of nucleons in the nucleus. |
| Atomic number | The atomic number is the number of protons in the nucleus. |
| Neutron number | The neutron number is the number of neutrons in the nucleus. |
| Electron number | The electron number is equal to the atomic number of the element. |
| Magnetic moments | Both protons and neutrons have magnetic moments, but the nucleon magnetic moments are anomalous. |
| Stability | A proton by itself is thought to be stable, while a neutron in a free state is unstable. Inside a nucleus, combined protons and neutrons (nucleons) can be stable or unstable depending on the nuclide, or nuclear species. |
| Nuclear force | The interaction between two or more nucleons is called internucleon interaction or nuclear force, which is caused by the strong interaction. |
Explore related products
What You'll Learn

Nucleons are composite particles made of three quarks
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number. Nucleons are composite particles, each made of three quarks bound together by the strong interaction. This strong interaction is caused by the strong force, or gluons, which mediate the strong force at the quark level.
Until the 1960s, nucleons were thought to be elementary particles, not composed of smaller parts. However, they are now understood to be composite particles. Protons and neutrons, or nucleons, are made of three quarks each. A proton is composed of two up quarks and one down quark, while a neutron has one up quark and two down quarks. The slight difference in the masses of up quarks and down quarks composing the nucleons explains the small difference in the masses of protons and neutrons.
The quark composition of nucleons is reflected in their positive or neutral electric charge. Protons have a positive charge, with a quark composition of uud, while neutrons are neutral, with a quark composition of udd. These quark compositions also correspond to the number of up and down quarks in each nucleon. The magnetic moments of protons and neutrons, which were unexpected when discovered in the 1930s, provide strong evidence for the quark model of nucleons. These magnetic moments are consistent with the quark model and inconsistent with the predictions of a point particle model.
While nucleons are composed of three quarks, it is important to note that they also contain virtual gluons, which are implied in the strong interaction. Some perspectives view the proton as being made of thousands of quarks and anti-quarks that are constantly created and annihilated. From this perspective, the proton has three more quarks than anti-quarks at any given instant. However, the cancelling quarks are considered real particles by some and virtual particles by others, highlighting the complexity of these subatomic structures.
The Magna Carta's Influence on the US Constitution
You may want to see also

The number of nucleons in a nucleus defines the atom's mass number
In physics and chemistry, a nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number. The mass number of a nucleus is equal to the number of neutrons in the nucleus, the number of protons in the nucleus, and the number of nucleons in the nucleus.
The mass number of an atom is denoted by the letter A and is the sum of the total number of protons and neutrons contained by the atom. The atomic number of an element is the net charge on the nucleus of the atom. Since neutrons are chargeless particles, the charge on the nucleus is due to the number of protons in the atom. Therefore, the atomic number is equal to the number of protons in the atom. The mass number is the sum of both protons and neutrons in the nucleus, and the atomic number is equal to the number of protons, so the mass number is usually twice the atomic number.
The nucleon number of an atom can be calculated by adding the number of protons and neutrons in the atom. For example, the atomic number (Z) of carbon is 6. The mass number (A) of carbon is 12. Therefore, the nucleon number of C-atoms is 12. Similarly, the mass number of chlorine is 35, so the nucleon number of chlorine is 35.
The nucleon number of an atom can also be determined by its atomic and mass numbers. The atomic number is the number of protons in the atom, and the mass number is the total number of protons and neutrons in the atom. By subtracting the atomic number from the mass number, the number of neutrons in the atom can be determined. The nucleon number is the sum of the number of protons and neutrons in the atom.
Nucleons were once thought to be elementary particles, but they are now understood as composite particles made of three quarks bound together by the strong interaction. The interaction between two or more nucleons is called internucleon interaction or nuclear force, which is ultimately caused by the strong interaction.
The French Constitution of 1791: Democracy or Not?
You may want to see also

Protons and neutrons are nucleons, electrons are not
In physics and chemistry, a nucleon is defined as a proton or a neutron that forms part of an atomic nucleus. The number of nucleons in a nucleus defines the atom's mass number. Protons and neutrons are bound together by the strong interaction, which is a result of particle physics and nuclear physics. This interaction between nucleons is called internucleon interaction or nuclear force.
Protons and neutrons are nucleons, while electrons are not. This is because electrons do not form part of the atomic nucleus. Instead, electrons surround the nucleus. Protons have a positive charge, electrons have a negative charge, and neutrons have no charge. The charges on protons and electrons are the same size but opposite, which is why opposite charges attract, and protons and electrons attract each other. This attraction holds an atom together.
The mass of a proton is 1.6726x10^-27 kg, while the mass of a neutron is 1.6749x10^-27 kg. This means that the neutron is roughly 0.13% heavier than the proton. The similarity in mass between protons and neutrons can be explained by the slight difference in masses of up quarks and down quarks, which compose nucleons. However, a detailed description of this remains an unsolved problem in particle physics.
Protons and neutrons can be stable or unstable depending on the nuclide or nuclear species. A neutron in a free state is an unstable particle, with a half-life of around ten minutes. It undergoes beta-minus decay, a type of radioactive decay, by turning into a proton and emitting an electron and an electron antineutrino. This occurs because the mass of the neutron is slightly greater than that of the proton. On the other hand, a proton by itself is thought to be stable, with a lifetime too long to measure. Inside a nucleus, protons and neutrons can combine to form stable or unstable particles, depending on the nuclear species.
The Social Contract: US Constitution's Core Principle
You may want to see also
Explore related products
$10.13

Nucleons have magnetic moments
The number of nucleons in a nucleus defines an atom's mass number. A nucleon is either a proton or a neutron, which are considered to be components of an atomic nucleus. Both protons and neutrons have magnetic moments, though nucleon magnetic moments were unexpected when they were discovered in the 1930s. The proton magnetic moment was discovered in 1933, and the neutron was discovered in 1932. Since neutrons have no charge, it was assumed they had no magnetic moment. However, indirect evidence suggested that neutrons had a non-zero value for their magnetic moment, which was proven by direct measurements in 1940.
The magnetic moment of the electron is about 1000 times larger than that of nucleons. The magnetic moments of antiprotons and antineutrons have the same magnitudes as their antiparticles, but they have an opposite sign. The nucleon magnetic moments arise from the quark substructure of nucleons. The nucleons are made up of three quarks, and the magnetic moments of these elementary particles combine to give the nucleons their magnetic moments. The magnetic moment of a nucleus with an even number of protons and neutrons is zero, while a nucleus with an odd number of protons and an even number of neutrons will have the magnetic moment of the remaining unpaired nucleon.
The nuclear magnetic moment is the magnetic moment of an atomic nucleus and arises from the spin of the protons and neutrons. It is a magnetic dipole moment, and all nuclei with nonzero spin also have a nonzero magnetic moment. The nuclear magnetic moment varies from isotope to isotope, and the nuclear spin and magnetic moment are always zero when the numbers of protons and neutrons are both even in the lowest energy state. In cases with odd numbers of protons and neutrons, the nucleus often has nonzero spin and magnetic moment.
The nucleons have spin angular momentum, and this causes them to precess with a well-defined frequency, called the Larmor frequency. This phenomenon enables the measurement of nuclear properties through nuclear magnetic resonance. The Larmor frequency can be determined from the product of the gyromagnetic ratio with the magnetic field strength. The sign of γn is negative for neutrons, so the neutron's spin angular momentum precesses counterclockwise about the direction of the external magnetic field. Nuclear magnetic resonance employing the magnetic moments of protons is used for nuclear magnetic resonance (NMR) spectroscopy.
Electoral College: Constitutional or Not?
You may want to see also

Nuclear force is caused by the interaction between nucleons
Nuclear force, or nucleon–nucleon interaction, is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. The nuclear force binds nucleons into atomic nuclei. The number of nucleons in a nucleus defines the atom's mass number.
The discovery of the neutron in 1932 revealed that atomic nuclei were made of protons and neutrons, held together by an attractive force. By 1935, the nuclear force was conceived to be transmitted by particles called mesons. This theoretical development included a description of the Yukawa potential, an early example of a nuclear potential. The modern perception of the nuclear force is that it is a residual interaction of the even stronger force between quarks, which is mediated by the exchange of gluons and holds the quarks together inside a nucleon.
The nuclear force is powerfully attractive between nucleons at distances of about 0.8 femtometers (fm), but it rapidly decreases to insignificance at distances beyond 2.5 fm. At distances less than 0.7 fm, the nuclear force becomes repulsive. This repulsion is responsible for the size of nuclei, since nucleons can come no closer than the force allows. The force is stronger for particles with their spins aligned than for those with their spins anti-aligned.
Nuclear force is about 10 million times stronger than the chemical binding that holds atoms together in molecules. This is why nuclear reactors produce about a million times more energy per kilogram of fuel as compared to chemical fuel. However, the range of the nuclear force is short, only a few femtometers, beyond which it decreases rapidly. That is why, in spite of its enormous strength, we do not feel anything of this force on the atomic scale or in everyday life.
Congress Powers: 5 Key Abilities of US Lawmakers
You may want to see also
Frequently asked questions
A nucleon is either a proton or a neutron, considered in its role as a component of an atomic nucleus.
The mass number of a nucleus is the sum of the number of protons and neutrons in the nucleus.
The atomic number of an element is the number of protons in the nucleus. It is also equal to the net charge on the nucleus of the atom.
The nucleon number of an atom is the total number of protons and neutrons in the atom. It is also known as the mass number.
Protons and neutrons are both subatomic particles that reside inside the nucleus of an atom. Protons carry a positive net charge, while neutrons carry a zero net charge. Protons are considered stable, while free neutrons are unstable with a half-life of around ten minutes.







![By Catherine Anderson - Silver Thaw: A Mystic Creek Novel (2015-01-21) [Mass Market Paperback]](https://m.media-amazon.com/images/I/01RmK+J4pJL._AC_UY218_.gif)



































