Stereoisomers are distinguished based on their optical ability to deviate light, known as the R/S optical rotation system. This system was developed by chemists R.S. Cahn, C. Ingold, and V. Prelog, and it assigns priority to the different groups connected to the central carbon atom. The priority of a group is based on the atomic number of the atoms directly connected to the central carbon, with substituents of higher atomic numbers taking precedence over those with lower atomic numbers. Hydrogen, for example, has the lowest atomic number and thus the lowest priority. When determining the priority of stereoisomers, it is also important to consider the number of substituents, with higher priority assigned to atoms with more substituents of the same priority. Additionally, the direction of the arrow formed by connecting the highest to the second-highest ranked substituents determines whether the stereoisomer is classified as S (counterclockwise) or R (clockwise).
| Characteristics | Values |
|---|---|
| Basis for priority | Atomic number of the atoms directly connected to the central carbon |
| Lowest priority atom | Hydrogen |
| Higher priority | Higher atomic number |
| Higher priority | Higher mass number (when comparing isotopes) |
| Higher priority | More substituents (if two atoms have substituents of the same priority) |
| Configuration | S (if the arrow from the highest priority group to the second-highest moves in a counterclockwise direction) |
| Configuration | R (if the arrow moves in a clockwise direction) |
Explore related products
What You'll Learn

Hydrogen is the lowest priority atom
The Cahn-Ingold-Prelog rules, created by R.S. Cahn, C. Ingold, and V. Prelog, are used to distinguish stereoisomers based on their optical ability to deviate light. This is often referred to as the R/S optical rotation system.
To classify a stereoisomer, priority must be assigned to the different groups connected to the central carbon. A substituent with a higher atomic number takes precedence over a substituent with a lower atomic number. Hydrogen is the lowest possible priority atom because it has the lowest atomic number. When comparing isotopes, the atom with the higher mass number has higher priority.
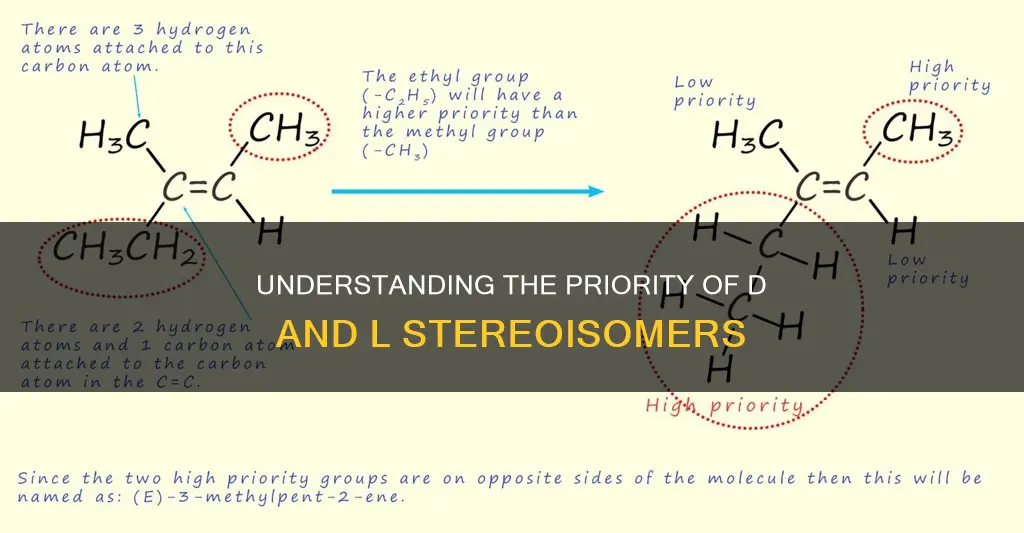
For example, an ethyl substituent takes precedence over a methyl substituent. At the connectivity of the stereocenter, both have a carbon atom, which are equal in rank. Going down the substituent chains, a methyl only has hydrogen atoms attached to it, whereas an ethyl has a carbon atom in addition to hydrogen atoms. The carbon atom has a higher atomic number than hydrogen, so ethyl takes priority over methyl.
Similarly, an alkene substituent (CH2=CH-) has a higher priority than an ethyl substituent (CH3CH2-) because the alkene carbon has "two" bonds to carbon atoms and one bond to a hydrogen atom, while the ethyl carbon only has one bond to a carbon atom and two bonds to hydrogen atoms.
When determining the configuration of a stereoisomer, the arrow from the highest priority group to the second-highest is drawn. If the arrow moves in a counterclockwise direction, the stereoisomer is classified as S. If it moves in a clockwise direction, it is classified as R.
Wealth, Income, and the Upper Middle Class in America
You may want to see also

Higher atomic numbers denote higher priority
The Cahn–Ingold–Prelog ?(CIP) sequence rules, also known as the CIP priority convention, are a set of rules used in organic chemistry to name and distinguish stereoisomers. The rules were created by chemists R.S. Cahn, C. Ingold, and V. Prelog. This set of rules distinguishes enantiomers based on their optical ability to deviate light and is referred to as the R/S optical rotation system.
Before a stereoisomer can be classified as either S or R, priority must be assigned to the different groups connected to the central carbon. The priority is based on the atomic number of the atoms directly connected to the central carbon. A substituent with a higher atomic number takes precedence over a substituent with a lower atomic number. Hydrogen has the lowest atomic number and, therefore, the lowest priority.
When dealing with isotopes, the atom with the higher atomic mass receives higher priority. In cases where two atoms have substituents of the same priority, a higher priority is assigned to the atom with more of these substituents. The molecule should then be rotated so that the group with the lowest priority is directed away from the viewer.
Once the priorities have been assigned and the molecule oriented, the stereoisomer can be classified. If the arrow from the highest priority group to the second-highest moves in a counterclockwise direction, the stereoisomer is classified as S. If the arrow moves in a clockwise direction, it is classified as R.
The CIP system provides a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond. This allows for the unique specification of the configuration of the entire molecule by including these descriptors in its systematic name.
Framers' Constitution Approach: A Historical Perspective
You may want to see also

Stereoisomers are classified as S or R
Stereoisomers are molecules with the same molecular constitution but a different three-dimensional spatial arrangement of atoms. They can be classified as enantiomers or diastereomers. Enantiomers are stereoisomers that form mirror images of each other, like a pair of human hands. Diastereomers, on the other hand, are stereoisomers that do not form mirror images.
When the mirror images of two stereoisomers are not superimposable, they are termed chiral. Such molecules have one or more carbon atoms, also known as chiral or stereocentres, with four non-identical substituents. The substituents are arranged in a way that prevents the superimposition of mirror images, even though the substituents attached are the same.
The R and S nomenclature system, also known as the Cahn-Ingold-Prelog rules, is used to name and distinguish these stereoisomers. This system is based on the optical ability of the stereoisomers to deviate light and is referred to as the R/S optical rotation system. Stereoisomers are classified as S or R depending on the direction in which the arrow, drawn from the highest priority group to the second-highest priority group, moves. If the arrow moves in a counterclockwise direction, the stereoisomer is classified as S, while a clockwise direction results in an R classification.
The priority of the groups connected to the central carbon is determined by the atomic number of the atoms directly connected to it. A substituent with a higher atomic number takes precedence over one with a lower atomic number. For example, a 1-methylethyl substituent takes precedence over an ethyl substituent because it has two carbon atoms attached to the first carbon atom, whereas ethyl only has one. Hydrogen has the lowest priority. If two atoms have substituents of the same priority, the atom with more of these substituents is given higher priority.
The R and S nomenclature does not identify the optical rotation of a compound as being either positive or negative. The direction of optical rotation is determined experimentally using a polarimeter. The absolute configuration is determined by X-ray crystallography or X-ray diffraction analysis.
Understanding California's Assignment of Contract Rules
You may want to see also
Explore related products

Prioritise atoms with more substituents
When determining the priority of stereoisomers, it is essential to follow a set of rules, often referred to as the Cahn-Ingold-Prelog rules, which distinguish enantiomers based on their optical ability to deviate light. These rules outline a systematic approach to prioritising atoms and their substituents.
The first step is to examine the atoms directly attached to the stereocenter, or chiral centre, of the compound. The atom with the higher atomic number takes precedence over atoms with lower atomic numbers. For example, iodine (I) has a higher priority than bromine (Br), which in turn has a higher priority than hydrogen (H), which is the lowest possible priority atom due to its low atomic number. When comparing isotopes, the atom with the higher mass number is given higher priority.
In cases where two atoms have substituents of the same priority, the atom with more of these substituents is assigned a higher priority. This means that if one atom has multiple substituents of higher priority compared to another atom with fewer of these substituents, the atom with more substituents will be prioritised. For instance, an ethyl substituent (CH3CH2-) has a higher priority than a methyl substituent because, in addition to its carbon atom, it has two hydrogen atoms, whereas a methyl substituent only has hydrogen atoms attached to it.
It is important to note that when dealing with multiple bonds, each bond is treated as if it is bonded to a unique atom. For example, an alkyne substituent (HCC-) has a higher priority than an alkene substituent (CH2=CH-) because the alkyne carbon is treated as if it is bonded to three carbon atoms.
Once the priorities of the substituents have been determined, the molecule should be rotated in space so that the group with the lowest priority is directed away from the viewer. This ensures that the molecule is in the correct orientation for further analysis.
By following these rules and assigning priorities to the different groups connected to the central carbon, a stereoisomer can be classified as either R or S configuration. If the arrow from the highest priority group to the second-highest moves in a counterclockwise direction, the stereoisomer is classified as S. If the arrow moves in a clockwise direction, it is classified as R.
The Constitution: Guarding Against Tyranny
You may want to see also

Stereoisomers are named and distinguished using Cahn-Ingold-Prelog rules
Stereoisomers are molecules that are stereoisomers that are non-superimposable mirror images of each other. They rotate plane-polarized light in equal and opposite directions. A tetrahedral atom with four different substituents (a chiral centre) can have two different configurations.
In 1966, Robert Cahn, Chris Ingold, and Vladimir Prelog developed a naming scheme for stereoisomers called the Cahn-Ingold-Prelog (CIP) rules. The CIP rules are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers.
The Cahn-Ingold-Prelog rules are based on the priority of the four groups around a chiral centre. The four substituents are assigned priorities based on their atomic numbers, with the highest atomic number assigned priority #1, and the lowest assigned priority #4. The molecule is then oriented in space so that the group with the lowest priority is pointed away from the observer. If the substituents are numbered from 1 (highest priority) to 4 (lowest priority), then the sense of rotation of a curve passing through 1, 2 and 3 distinguishes the stereoisomers. An arc drawn clockwise is assigned rectus (R) and an arc drawn counterclockwise is assigned sinister (S). The names are derived from the Latin for 'right' and 'left', respectively.
For double-bonded molecules, the Cahn-Ingold-Prelog priority rules are followed to determine the priority of substituents of the double bond. If both of the high-priority groups are on the same side of the double bond (cis configuration), then the stereoisomer is assigned the configuration Z (from the German word "zusammen" meaning "together"). If the high-priority groups are on opposite sides of the double bond (trans configuration), then the stereoisomer is assigned the configuration E (from the German word "entgegen" meaning "opposed").
The Cahn-Ingold-Prelog rules provide a method for unambiguously assigning the handedness of molecules. In addition to the Cahn-Ingold system, there are two ways of experimentally determining the absolute configuration of an enantiomer: X-ray diffraction analysis and chemical correlation with a molecule whose structure has already been determined via X-ray diffraction.
Locke's Influence on the Constitution
You may want to see also
Frequently asked questions
The rules for prioritising stereoisomers, also known as the Cahn-Ingold-Prelog rules, state that priority is determined by the atomic number of the atoms directly connected to the central carbon. A substituent with a higher atomic number takes precedence over one with a lower atomic number.
The priority rankings of atoms are as follows: I > Br > Cl > S > P > F > O > N > C > H. Hydrogen has the lowest atomic number and is therefore the lowest priority atom.
If two atoms have substituents of the same priority, then a higher priority is given to the atom with more of these substituents.
To determine the configuration of a stereoisomer, you must first assign priority to the different groups connected to the central carbon. If an arrow from the highest priority group to the second highest moves in a counterclockwise direction, the stereoisomer is classified as S. If the arrow moves in a clockwise direction, the stereoisomer is classified as R.










































